Dental calculus (DC) is a common deposit in periodontitis patients. DC contains both microbial components and calcium phosphate crystals that induce an osteoclastogenic cytokines, such as IL-1β and IL-18, via the NLRP3 inflammasome in macrophages.
1. Introduction
Periodontitis is an inflammatory disease that leads to the destruction of periodontal tissue, including alveolar bone
[1]. In response to external stimuli, such as dental plaque and dental calculus (DC), various types of leukocytes infiltrate periodontal tissue, and release inflammatory mediators, such as prostaglandins, matrix metalloproteinases, and cytokines, which promote periodontal tissue destruction
[2]. Among these mediators, interleukin (IL)-1β has the capacity to trigger potent bone resorption
[3], and IL-1β has been detected in the periodontal tissue and gingival crevicular exudates of patients with periodontitis, suggesting its involvement in alveolar bone resorption
[4][5][4,5].
The production of IL-1β is regulated both transcriptionally and post-transcriptionally
[6]. The transcription of pro-IL-1β can be triggered by the binding of Toll-like receptors (TLRs), IL-1 receptors, and tumor necrosis factor (TNF) receptors with their ligands, leading to the nuclear translocation of nuclear factor (NF)-κB
[7]. The transcriptional activation of the IL-1β promoter results in the synthesis of a 35 kD pro-IL-1β, which is biologically inactive and remains in the cytosol
[8]. For the maturation of IL-1β, pro-IL-1β must be processed by caspase-1
[9]. Assembly of the inflammasome, which consists of nucleotide-binding oligomerization domain leucine-rich repeat containing protein (NLR), apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC), and the cysteine protease pro-caspase-1, converts pro-caspase-1 to active caspase-1 by autocatalysis; caspase-1, in turn, cleaves pro-IL-1β into its mature IL-1β form
[10].
IL-18 and IL-33 are also IL-1 family members that can be cleaved by caspase-1
[11][12][11,12]. IL-18 is known to be a proinflammatory cytokine that induces the production of interferon (IFN)-γ in T cells
[13], and promotes osteoclastogenesis by upregulating receptor activator of nuclear factor-kappa B ligand (RANKL) production in T cells in synovitis in rheumatoid arthritis
[14]. IL-33 binds to type 4 IL-1 receptors and is regarded as a Th2-promoting inflammatory cytokine
[15]. The precursor form of IL-33 is already biologically active and is inactivated via cleavage by caspase-1
[11][16][11,16]. IL-33 has been reported to inhibit osteoclastogenesis
[17][18][17,18].
DC is a deposit frequently found in periodontal pockets, and is 70–80% inorganic material
[19]. The remaining organic component consists of proteins, leukocytes, and microorganisms
[20]. The crystalline components of DC are mainly hydroxyapatite, brushite, tricalcium phosphate, and octacalcium phosphate
[19][21][19,21]. Recent studies have shown that stimulation of neutrophils and macrophages with DC activates the nucleotide-binding oligomerization domain leucine-rich repeat and pyrin domain containing 3 (NLRP3) inflammasome and produces IL-1β
[22]. In addition, IL-18 and IL-33 can be produced by mouse macrophages stimulated with DC via the NLRP3 inflammasome. However, the effects of these cytokines stimulated with DC on osteoclastogenesis have not been investigated.
It is difficult to distinguish the effects of DC from those of dental biofilms because DC is always covered with biofilms that contain highly viable bacteria
[23]. However, accumulated evidence has shown that there is a clear association between DC deposition and periodontitis
[23][24][23,24].
2. Effects of Culture Supernatants from Mouse Macrophages Stimulated with Dental Calculus on Osteoclast Formation
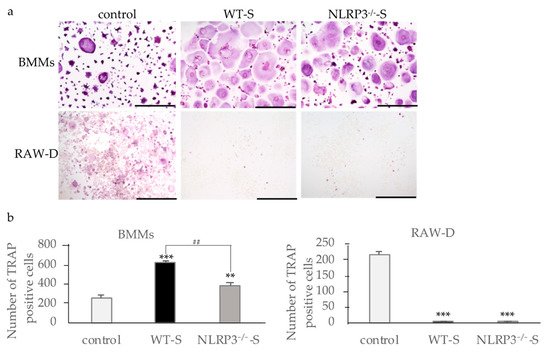
When bone marrow macrophages (BMMs) primed with M-CSF and RANKL were incubated with the supernatant from the NLRP3-deficient mouse macrophages stimulated with DC, osteoclast formation was promoted, suggesting the effects of pro-inflammatory cytokines in the supernatants (Figure 1a). The supernatant of WT mouse macrophages stimulated with DC induced higher numbers of osteoclasts than the supernatant from NLRP3-deficient mouse macrophages, suggesting the effect of another pro-inflammatory cytokine (Figure 1b). In contrast to the results of RANKL-primed BMMs, the supernatants from both WT and NLRP3-deficient mouse macrophages stimulated with DC suppressed osteoclast formation in RANKL-primed RAW-D cells (Figure 1a,b). Incubation of RANKL-primed RAW-D cells with the supernatant from unstimulated WT or NLRP3-deficient mouse macrophages did not affect the osteoclastogenesis.
Figure 1. Effects of culture supernatants from mouse macrophages stimulated with dental calculus (DC) on osteoclastogenesis. BMMs were incubated with 30 ng/mL M-CSF and 20 ng/mL RANKL for 48 h. Then, the cells were incubated for 24 h with the same concentration of M-CSF, RANKL, and the culture supernatant from WT or NLRP3−/− mouse macrophages stimulated with DC. RAW-D cells were incubated with 20 ng/mL RANKL for 48 h. The cells were then incubated for 48 h with the same concentration of RANKL and the culture supernatant from WT or NLRP3−/− mouse macrophages stimulated with DC. These cells were subjected to TRAP staining (a), and TRAP-positive cells with more than three nuclei were counted (b). Scale bar = 500 μm. The differences between the groups were analyzed by one-way ANOVA followed by a Tukey test for multiple comparisons. ** p < 0.01 *** p < 0.001 compared with the control. ## p < 0.01 compared among the test groups. BMM, bone marrow macrophages; DC, dental calculus; M-CSF, macrophage colony-stimulating factor; NLRP3−/−-S, culture supernatant of the NLRP3-deficient mouse macrophages stimulated with DC; WT, wild-type; WT-S, culture supernatant of the wild-type mouse macrophages stimulated with DC.
3. Cytokine Production by Macrophages Stimulated with DC
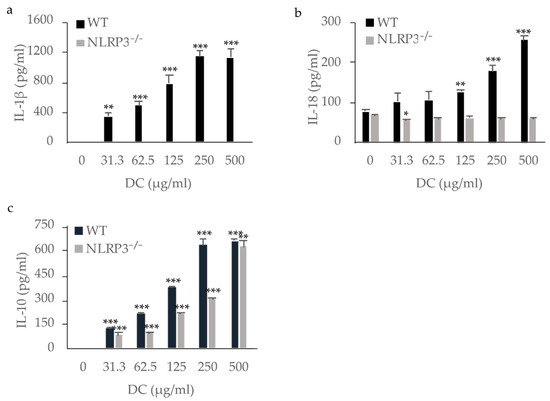
WT mouse macrophages produced IL-1β in a dose-dependent manner, whereas NLRP3-deficient mouse macrophages did not. This indicates that IL-1β was produced via NLRP3 by mouse macrophages stimulated with DC (
Figure 23a). Although IL-18 was produced by unstimulated WT mouse macrophages, stimulation with DC enhanced its production (
Figure 23b). Stimulation of NLRP3-deficient mouse macrophages with DC resulted in no increase in IL-18 production, indicating that IL-18 production was induced via NLRP3 in mouse macrophages stimulated with DC. IL-33, another IL-1 family member possibly processed by caspase-1
[11][16][11,16], was not detected in the supernatants of either WT or NLRP3-deficient mouse macrophages (data not shown). IL-10, an anti-inflammatory cytokine, was produced by both WT and NLRP3-deficient mouse macrophages stimulated with DC in a dose-dependent manner, indicating the production of IL-10 independently of the NLRP3 inflammasome (
Figure 23c). Tumor necrosis factor (TNF)-α, a pro-inflammatory cytokine, was also produced by both WT and NLRP3-deficient mouse macrophages stimulated with DC (data not shown).
Figure 23. Production of IL-1β, IL-18, and IL-10 by mouse macrophages stimulated with dental calculus (DC). WT and NLRP3
−/− mouse macrophages were stimulated with DC for 8 h. The production of IL-1β (
a), IL-18 (
b), and IL-10 (
c) were measured by ELISA. The differences between the groups were analyzed by one-way ANOVA followed by a Tukey test for multiple comparisons. *
p < 0.05 **
p < 0.01 ***
p < 0.001 compared with the unstimulated control. WT, wild-type.
4. Effects of IL-1β, IL-18, and IL-10 on Osteoclast Formation
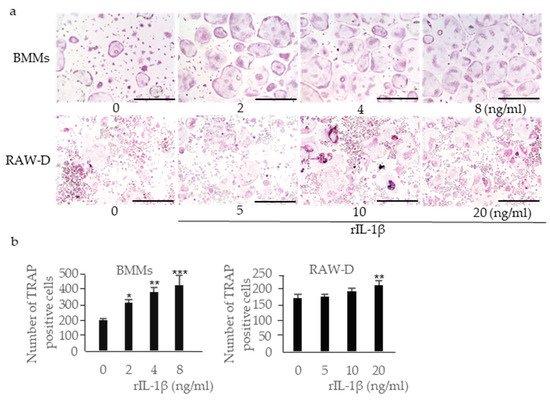
Incubation of RANKL-primed BMMs with rIL-1β increased the number of multinucleated TRAP-positive cells in a dose-dependent manner (
Figure 35a,b). Incubation of RANKL-primed RAW-D cells with rIL-1β also increased the number of multinucleated TRAP-positive cells, although the effect was less prominent. IL-18 is classified as a pro-inflammatory cytokine; however, previous studies have shown that it inhibits osteoclast formation by inducing osteoclast precursor cell apoptosis
[25]. Incubation of RANKL-primed BMMs with rIL-18 significantly reduced the number of multinucleated TRAP-positive cells (
Figure 46a,b). Incubation of RANKL-primed RAW-D cells with rIL-18 also reduced the number of multinucleated TRAP-positive cells, although the effect was less prominent. IL-10 is an anti-inflammatory cytokine that inhibits osteoclast formation
[26]. Incubation of RANKL-primed BMMs with rIL-10 reduced the number of multinucleated TRAP-positive cells, and incubation of RANKL-primed RAW-D cells with rIL-10 reduced the number of multinucleated TRAP-positive cells even more effectively (
Figure 46c,d). Because the inhibitory effect of rIL-10 in RAW-D cells was considerably stronger than that in RANKL-primed BMMs, we analyzed the expression of IL-10 receptors in these cells. The expression of both IL-10 receptor α and β genes (
IL10RA and
IL10RB, respectively) was significantly downregulated after the priming with RANKL in RAW-D cells, but not in BMMs, consistent with higher sensitivity of RANKL-primed RAW-D cells to IL-10 (
Figure 46e).
Figure 35. Effects of recombinant (r)IL-1β on osteoclastogenesis. BMMs were incubated with 30 ng/mL M-CSF and 20 ng/mL RANKL for 48 h. Then, the cells were incubated for 24 h with the same concentration of M-CSF, RANKL, and rIL-1β. RAW-D cells were incubated with 20 ng/mL RANKL for 48 h. Then, the cells were incubated for 48 h with the same concentration of RANKL and rIL-1β. These cells were subjected to TRAP staining (
a), and TRAP-positive cells with more than three nuclei were counted (
b). Scale bar = 500 μm. The differences between the groups were analyzed by one-way ANOVA followed by a Tukey test for multiple comparisons. *
p < 0.05 **
p < 0.01 ***
p < 0.001 compared with controls containing no rIL-1β. BMM, bone marrow macrophage; M-CSF, macrophage colony-stimulating factor; TRAP, tartrate-resistant acid phosphatase.
Figure 46. Effects of recombinant (r)IL-18 and rIL-10 on osteoclastogenesis. BMMs were incubated with 30 ng/mL M-CSF and 20 ng/mL RANKL for 48 h. Then, the cells were incubated for 24 h with the same concentration of M-CSF, RANKL, and rIL-18 (
a,
b) or rIL-10(
c,
d). RAW-D cells were incubated with 20 ng/mL RANKL for 48 h. Then, the cells were incubated for 48 h with the same concentration of RANKL and rIL-18 or rIL-10. These cells were subjected to TRAP staining (
a,
c), and TRAP-positive cells with more than three nuclei were counted (
b,
d). Total RNA was extracted from the unstimulated and RANK-primed cells, and the relative expression of
IL-10RA and
IL-10RB mRNA was determined using quantitative reverse transcription–polymerase chain reaction (qRT–PCR) (
e). Scale bar = 500 μm. The differences between the groups were analyzed by one-way ANOVA followed by a Tukey test for multiple comparisons. The differences between two groups were analyzed using
t-tests. *
p < 0.05 **
p < 0.01 ***
p < 0.001 compared with the control. BMM, bone marrow macrophage; M-CSF, macrophage colony-stimulating factor; TRAP, tartrate-resistant acid phosphatase.