Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sandrine Gerber and Version 3 by Dean Liu.
Chitosan (CS) is a natural biopolymer that has gained great interest in many research fields due to its promising biocompatibility, biodegradability, and favorable mechanical properties. Through covalent and non-covalent chemical modifications, CS derivatives can reach optimal properties for the development of smart biomaterials in a wide range of biomedial applications.
- chitosan
- chemical modifications
- covalent conjugation
- non-covalent interactions
1. Strategies for Covalent Functionalization
The common methodologies for CS covalent modification rely on chemical derivatization of the primary amine and alcohol functionalities, or partial oxidative cleavage of the backbone. CS thiolation was also reported for several targeted applications and was recently reviewed by Federer et al. and Summonte et al. [1][2][24,25]. While CS is most often used as starting material, more advanced intermediates such as CMCS, TMC, or O-glycol CS (GCS) are also commercially available to facilitate downstream modifications (Figure 13). Of note, reacetylation of CS and, more specifically, GCS has also been reported in order to obtain O-glycol chitin [3][26].
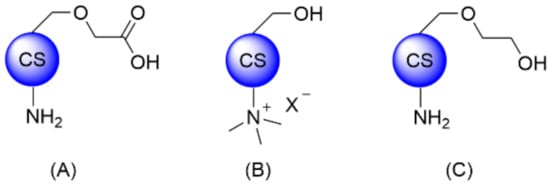
Figure 13. Structures of commercially available CS derivatives for further functionalization: (A) CMCS, (B) TMC, and (C) GCS.
2. Non-Covalent Modifications
CS-based materials have also been combined with small molecules, proteins, and polymers via non-covalent modifications. Under these conditions, the stability of the resulting CS derivatives relies on three main types of non-covalent interactions: H-bonding, electrostatic interactions (ionic bonds), and chelation (Figure 27A–C). Entanglement of larger molecules into a CS-based matrix was also reported and can add to the stabilization of the resulting hydrogel, as illustrated in Figure 27D.
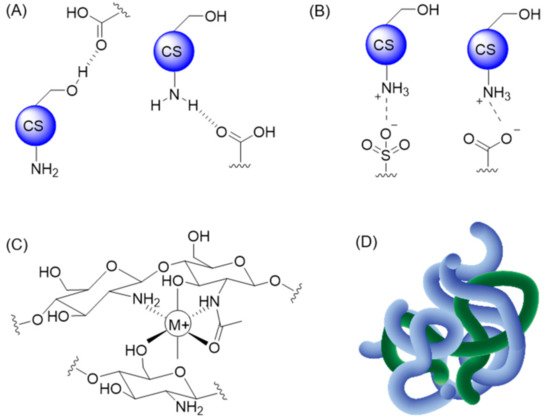
Figure 27. Examples of non-covalent interactions with CS: (A) H-bonding, (B) ionic interactions, (C) chelation, and (D) entanglement between multiple polymeric chains.
The assembly of CS derivatives through non-covalent modifications avoids the need for chemical reagents and thus simplifies purification procedures, which can be complex and time-consuming. While non-covalent conjugates are easily prepared, there is generally little control over their association and interaction patterns. In addition, their chemical characterization remains very challenging and does not provide precise information on the intermolecular organization and availability of functional moieties (see Section 5 for more details on characterization). This section focuses on solid CS-based materials (nanofibers, NPs, and dry films) or hydrogels. Interestingly, the mechanical resistance and stability of CS derivatives can be significantly improved by their non-covalent association to specific additives. This strategy was highlighted in bone tissue engineering and wound dressing applications [4][5][101,102] and is also suitable for the development of pH-responsive conjugates [6][103]. This section describes systems involving non-functionalized CS forming non-covalent interactions with other components including polymers, proteins, small molecules, and nanocomposites.
3. Combination of Covalent and Non-Covalent Modifications
In this section, we describe CS-based systems that present both covalent and non-covalent interactions. In these cases, the amino groups of CS units are usually functionalized with a small molecule or a polymer prior to mixing with another component, but a few examples of the reverse process were also disclosed. The resulting systems combine both covalent functionalization patterns, which are stable under a large variety of conditions, and non-covalent interactions, which are more prone to disruption depending on the environment. This dual conjugation strategy offers the opportunity to modulate the stability and hydrophilicity of the final CS-based materials. The main interest in associating the two types of interactions is that it combines the stability of simple covalent grafting with the ease of preparation of self-associating molecules. To the best of our knowledge, hydrophobically-modified CS derivatives were exclusively employed in association with single-walled or multi-walled carbon nanotubes (CNT) [7][8][145,146]. Consequently, this section describes almost exclusively hydrophilic systems. The nature of the interactions between functionalized CS and complementary molecules refers mostly to H-bonding and ionic interactions. In some cases, metal ion chelation and aromatic π–π stacking also participate in the stabilization of the system.
3.1. Functionalization Strategies of CS Intended to Non-Covalent Mixing
The first step toward dual covalent/non-covalent CS-based materials generally makes use of covalent functionalization of the reactive CS amines. This initial modification of the CS backbone can add valuable properties such as improved solubility and increased bio-adhesion [9][147] and biocompatibility [10][75] to the final material. The number of modified glucosamine units is commonly expressed as the grafting degree (GD). As the increased solubility of CS derivatives is known to result from the disturbance of the polymeric chain stacking by grafted molecules, CS amine functionalization is almost always associated with an improved solubility of CS in aqueous solution, independently of the type or role of the molecule grafted (whether sugar, alkyl, or small charged moieties). These conjugation reactions are considered to result in a homogeneous functionalization of the bulk CS polymer (Figure 39A). However, some studies took advantage of an outer-shell derivatization to locally functionalize CS reactive sites. More specifically, when CS was used as a coating agent, derivatization of the polymer was performed after the formation of a core–shell complex (Figure 39B). Shahdeo et al. made use of this strategy to introduce a cancer targeting peptide sequence on CS-coated Au NPs, thus ensuring the accessibility of the bioactive component by selectively anchoring it on the CS amines of the outer shell [11][148].
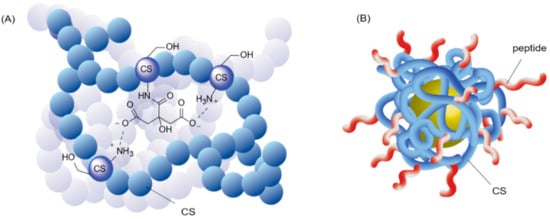
Figure 39. Illustrations of the difference between bulk and surface functionalization of CS hydrogels. (A) Interaction between citric acid (CA) and CS in bulk. Inspired from [12][149]. For visibility, the CS units not involved in the interaction are represented by a simple blue ball. CA is both covalently linked to a CS unit and engaged in ionic interactions with the surrounding glucosamine. (B) External peptide functionalization of a core-shell system of CS-Au NP. The peptides are covalently linked to the outer CS shell coating. In this case, the peptides are all oriented towards the outside of the complex. Inspired from [11][148].
CS quaternization was frequently applied to improve the solubility of the polymer in aqueous media by increasing the density of positive charges on the backbone and hence promoting electrostatic repulsions, as well as disturbing the intra- and extra-molecular H-bond network through the incorporation of lateral alkyl chains. Quaternization is commonly achieved through epoxide ring opening, quaternary ammonium substitution, and N-alkylation (See Section 2.1). The recent review by Andreica et al. details synthetic pathways for quaternary ammonium salt CS and physicochemical changes that occur upon its formation [13][150].
The preparation of amphiphilic CS co-polymers capable of self-assembling into micelles was developed over the last decade. These assemblies offer great promises as vectors for drug and gene delivery. Typically, one or more hydrophilic moieties (such as PEG and sulphate) are first grafted on the CS chain, followed by further functionalization with hydrophobic alkyl or aryl groups. The simultaneous presence of functional moieties and polymer with different water affinities provides the amphiphilic character to the final polymeric material, which assembles into micelles (Scheme 1Scheme 10) [14][15][151,152]. Applications to pH-responsive nanocarriers were reported in the context of controlled delivery of chemotherapeutics to tumor sites [16][17][90,153].
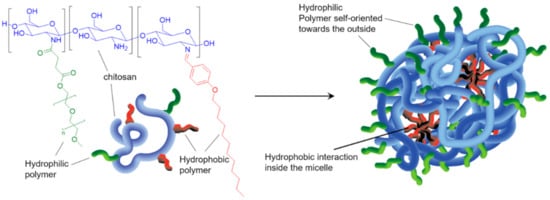
Scheme 10. Representative example of CS-copolymers. CS is first functionalized with methylated PEG (mPEG) and then with 4-(dodecyloxy)benzaldehyde (DBA). The resulting copolymer CS-mPEG-DBA (left) self-assembles into micelles (right) via electrostatic interactions and hydrophobic effect. The micelles can be loaded with drug of interest and respond to various stimuli. Illustration based on [14][151].
As mentioned in the Introduction, the use and application of CS-based polymers expand. Pre-functionalized CS derivatives such as CMCS or TMC are now also available at a reasonable price from commercial suppliers to ease the work of scientists.
3.2. Adjuvants Mixed with Functionalized CS
The properties of CS-based materials can be tuned both by covalent functionalization with small molecules and additional non-covalent combination with secondary components. Below, four categories of adjuvants will be discussed: polymers, small molecular entities, proteins, and nanocomposites.
