You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Thomas Smith.
Hypothermic and normothermic machine perfusion in kidney transplantation are purported to exert a beneficial effect on post-transplant outcomes compared to the traditionally used method of static cold storage. Kidney perfusion techniques provide a window for organ reconditioning and quality assessment.
- machine perfusion
- kidney
- HMP
- NMP
- regenerative medicine
- biotechnology
1. Introduction
Kidney transplantation is the most economical [1] and effective [2,3,4,5][2][3][4][5] therapy for patients with end-stage renal disease (ESRD). However, there is a worldwide shortage of suitable kidneys for transplantation [6]. Over the next decade, the incidence of chronic kidney disease (CKD) and ESRD is expected to increase considerably, with CKD due to become the fifth leading cause of death by 2040 [7]. Strategies that increase the number of kidneys available for transplantation or improve transplant success rates and outcomes are likely to have a considerable effect on global health.
Machine perfusion technologies have emerged as an important tool in tackling critical problems intrinsic to transplantation, such as ischaemia reperfusion injury (IRI) [7[7][8][9],8,9], poor post-transplant graft function [10,11,12][10][11][12] and reduced graft survival [10]. Understanding how machine perfusion ameliorates these problems and optimising these methods will likely further improve patient outcomes. Although the central goal of this research (i.e., increasing the availability and quality of transplant kidneys) is uniform, the means by which this could potentially be achieved differs. Optimisation of machine perfusion technologies may improve kidney transplantation in three ways:
- -
-
Improvement of transplant outcomes through delivery of therapeutic agents to repair and regenerate kidneys.
- -
-
Reduction in the number of discarded kidneys by developing robust techniques of organ assessment.
- -
-
Reduction in ischaemic injury during the preservation interval to improve the ‘shelf life’ of donated kidneys and increase the number available for transplant.
- Improvement of transplant outcomes through delivery of therapeutic agents to repair and regenerate kidneys.
- Reduction in the number of discarded kidneys by developing robust techniques of organ assessment.
- Reduction in ischaemic injury during the preservation interval to improve the ‘shelf life’ of donated kidneys and increase the number available for transplant.
2. Why Do We Need Organ Preservation? What Are the Factors Diminishing Kidney Quality?
Modifiable factors that have a key impact on pre-transplant kidney quality are the periods of ischaemia that occur prior to transplant and the reperfusion injury that occurs following transplant. These are illustrated in Figure 1 and described below.
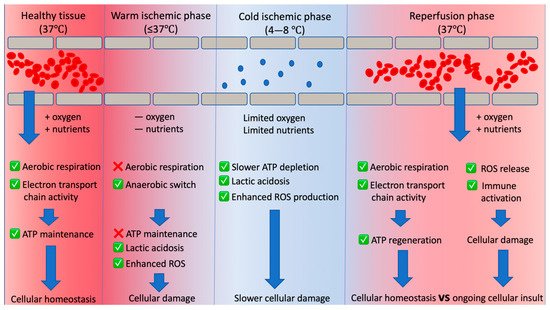

Figure 1. Overview of the changing graft tissue environment between organ donation and implantation. In health, homeostatic mechanisms ensure sufficient oxygen and nutrients are delivered to the renal tissue, resulting in balance between adenosine triphosphate (ATP) usage and regeneration. After donation, cessation of blood flow halts oxygen and nutrient supply (causing warm ischaemia). This causes an anaerobic switch that results in ATP depletion and accumulation of harmful metabolic by-products such as reactive oxygen species (ROS) and lactic acid. Cold ischaemia (chilling the organ) is deliberately implemented to slow the ATP depletion and damage that would occur under warm ischaemia. Restoration of blood flow drives ATP regeneration, but leads to another insult (ischaemia reperfusion injury) which occurs as a consequence of deleterious processes initiated by warm and cold ischaemia.
3. Warm Ischaemia
Preservation techniques maintain organ viability from the time of retrieval until transplantation. These techniques are required to counteract the destructive processes initiated during warm ischaemia. In general, warm ischaemia arises prior to donation [13] and results in impaired delivery of oxygen and metabolic substrates [14,15][14][15]. This drives an anaerobic shift [14] and crucially ATP depletion [16], which results in widespread deterioration of tissue structure [17,18,19,20,21,22][17][18][19][20][21][22]. The warm ischemic injury incurred also stimulates damaging inflammatory responses [23].
The warm ischaemic time (WIT) is associated with increased incidences of delayed graft function (DGF) [24] and therefore, the initial role of kidney preservation is to reduce ATP depletion, cell swelling and hypoxic injury. This is achieved by rapidly flushing the kidney at procurement with a cold preservation solution to slow metabolism and requirements for ATP.
4. Cold Ischaemia
Although effective in reducing metabolism, ongoing depletion of ATP leads to cold ischaemic damage. The cold ischaemic time (CIT) is an independent risk factor for the development of DGF [25]. The mechanisms of damage conferred under conditions of cold ischaemia have been described elsewhere [26].
5. Current Kidney Preservation Methods, Their Advantages and Limitations
5.1. Static Cold Storage
Static cold storage (SCS) is a simple and economical method of kidney preservation. Kidneys are placed in a bag of preservation solution and packed in wet ice, lowering the temperature to around 4 °C. At this temperature, enzymatic activity is reduced by approximately 58% [27]. Different preservations solutions are available, but University of Wisconsin (UW) solution is deemed the gold standard [27]. An overview of solutions used in SCS and the perfusion technologies described below is given in Table 1.
Table 1. Constituents of kidney preservation solutions in clinical use.
| SCS Fluids | HMP Fluids | NMP Fluids | |||
|---|---|---|---|---|---|
Table 2. Recent clinical trials optimising pretransplant kidney storage and machine perfusion protocols.
| NMP Clinical Trials | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NCT Number | Title | Primary Outcome Measure | Start Date | Completion Date | ||||||
| University of Wisconsin (UW) solution | Custodial-N solution | UW Machine perfusion solution (UWPS) | Hosgood protocol [28] | Minor protocol [ | ||||||
| NCT05031052 | Normothermic machine perfusion (NMP) vs Static Cold Storage (SCS) in Human Kidney transplantation | Kidney function at 6 months post-transplant (eGFR) | August 2021 | 29 | ] | |||||
| December 2025 | Base fluid | Water | Water | Water | Ringer’s solution | Steen solution Ringer’s solution |
||||
| NCT04882254 | Normothermic Machine Perfusion: An Additional Value for Kidney Transplant Outcomes? | Number of patients with immediate graft function within three months post-transplant | May 2021 | February 2023 | Volume expanders/osmotic agents | Hydroxyethyl starch | ||||
| NCT03136848 | Raffinose pentahydrate |
Mannitol | The Feasibility and Safety of Normothermic ex Vivo Kidney Perfusion |
| Hydroxyethyl starch Mannitol (USP) Magnesium gluconate Sodium gluconate |
Mannitol | Calcium gluconate | |||
| December 2016 | April 2019 | Oxygen carriers | - | - | - | 1 unit red blood cells (group O) | - | |||
| NCT04693325 | PROlonged Ex-vivo Normothermic Machine PERfusion for Kidney Regeneration | Glomerular filtration rate (GFR) at: 6 months post-transplantation | February 2021 | July 2022 | Drugs | Allopurinol Magnesium sulphate heptahydrate Lactobionic acid |
Deferoxamine | Dexamethasone Heparin Prostacyclin Insulin |
Ampicillin | |
| Antioxidants | ||||||||||
| NCT02525510 | Deceased Organ Donor Interventions to Protect Kidney Graft Function | Delayed Graft Function incidence within 1 week of transplantation | August 2017 | March 2022 | Glutathione | Tryptophan | Glutathione | |||
| ISRCTN15821205 | Ex Vivo Normothermic machine perfusion Trial | Delayed Graft Function incidence within 1 week of transplantation | January 2017 | |||||||
| - | Metabolic support |
Adenosine | Potassium hydrogen 2-ketoglutarate Sucrose Aspartate Arginine Alanine Glycine |
Glucose, beta D (+) Ribose |
Glucose, beta D (+) Synthamin 17 Cernevit multivitamins |
- | ||||
| Individual electrolyte additives |
- | Magnesium chloride Calcium chloride Potassium chloride Sodium chloride |
Calcium chloride | - | - | |||||
| Buffering agents | Potassium dihydrogen phosphate | Histidine Histidine · HCI |
HEPES (free acid) Potassium phosphate (monobasic) |
Sodium bicarbonate | Sodium bicarbonate | |||||
| pH adjustment | Sodium hydroxide/hydrochloric acid Potassium hydroxide |
- | Sodium hydroxide | - | - | |||||
5.2. Hypothermic Machine Perfusion
Hypothermic machine perfusion (HMP) involves the circulation of cold preservation fluid through the kidney using a mechanical pump. With the exception of several recent clinical trials, clinical HMP does not utilise active oxygenation. The limited metabolic support provided in the perfusion fluid was thought to be sufficient to meet the residual aerobic requirements under hypothermia [18].
ROS are a primary driver of reperfusion injury [30], and commonly used preservation fluids contain potent antioxidants, such as glutathione, to combat ROS activity during preservation. However, extended CIT is associated with marked perfusate glutathione depletion [31].
Machine perfusion solutions provide low-level metabolic support and antioxidant protection throughout perfusion. A key difference between HMP and SCS is the provision of fluid flow, which facilitates nutrient supply, waste removal and a limited amount of tissue reoxygenation with the dissolved oxygen present in the perfusate.
There are several commercially available HMP devices. The Organ Assist Kidney Assist, Waters RM3 and Organ Recovery Systems Lifeport are pressure-controlled systems designed to limit mechanical damage to the kidney during perfusion. Several new devices have been trialled, such as the AirDrive system which includes an oxygenator [32]. A new two-pump perfusion device which circulates fluid through the kidney and also in the organ reservoir has been used to deliver clinical HMP [33,34][33][34].
A number of randomised controlled trials and a meta-analysis have shown superiority of HMP over SCS techniques in improving early and longer-term graft function; however, despite this evidence HMP has not gained wide acceptance in some countries.
The evidence base supporting the use of HMP for all deceased donor kidneys is growing, with benefits recently reported in the UK [35[35][36],36], France [37], Poland [38] and Brazil [39,40][39][40]. HMP can also improve renal function when the CIT is extended [35].
In extend criteria donor (ECD) kidneys, use of HMP enhances 1-year graft survival [41]. However, HMP has not shown a convincing benefit in prolonging longer-term graft survival [42]. The Netherlands is the first country to introduce HMP for all deceased donor kidneys as standard practice [43]. Other countries use HMP specifically for donation after circulatory death (DCD) kidneys, but this practice is not uniform.
5.3. Normothermic Machine Perfusion
Normothermic machine perfusion (NMP) is a relatively new technique of preservation in kidney transplantation. It is currently used in combination with hypothermic preservation strategies as a form of end graft reconditioning. During NMP, kidneys are perfused at near-physiological temperatures and pressures allowing cellular metabolism and function to be restored. In the 1980s, interest in NMP using oxygenated blood-based perfusion solutions started to emerge, and demonstrated that short intervals or an end period of NMP could replenish cellular ATP [44].
The first case of NMP in clinical practice was published in 2011 [45]. The recipient received a kidney from an ECD donor that had been rejected by five other transplant centres in the UK. The kidney underwent NMP for a short interval immediately before transplantation. The recipient did not require dialysis post-transplant and 10 years post-transplant has normal kidney function (personal communication). Subsequently a series of NMP in ECD kidneys demonstrated a remarkably low rate of DGF (11%) compared to SCS kidneys (37%).
More recently, NMP has been used to rescue kidneys that were deemed unsuitable for transplant due to inadequate in situ perfusion after retrieval. Both kidneys were transplanted successfully with immediate graft function following transplant [46]. Building on this work, the authors developed a scoring system which could be used to assess kidney quality prior to transplant [46,47][46][47]. A large multicentre clinical trial assessing the effects of 1 h NMP in DCD kidneys compared to SCS has been completed and is due to report this year [28].
NMP conditions are still being developed and have recently been used to counteract ‘rewarming injury’ which occurs during the warm reperfusion of cold stored grafts. In 2015, the Minor group demonstrated that gradual rewarming (controlled oxygenated rewarming (COR)) of cold stored kidney grafts using machine perfusion improve creatinine clearance and reduces apoptotic signalling when compared to cold stored controls [48].
Kidneys are rewarmed (8–35 °C) over a 1.5 h period to allow metabolic adaptation to the changing thermal environment. Building on this work, the same group trialled their method in the clinical setting, reporting immediate graft function and acceptable levels of creatinine clearance within 1 week of transplantation [29].
More recently, a porcine auto-transplantation model demonstrated that while 8h of NMP improves renal function compared to SCS, a similar improvement in renal function is observed when cold-stored kidneys are subjected to 2 h of COR [49]. The authors speculate that this may be a useful application given the current requirement for hypothermic storage in the logistics of organ transport.
The perfusates used in clinical NMP have been defined in Table 1. The Hosgood et al. protocol provides a more physiological environment, with multiple metabolic substrates and red cells as an oxygen carrier. This contrasts with the Minor protocol, which utilises an acellular perfusate based on Steen solution [29,50][29][50].
Adapted cardiac bypass technologies or other perfusion set-ups can be used for NMP. The Kidney Assist, a pressure-controlled system, is the only CE-marked device on the market.
There are some limitations of NMP compared to HMP. These include a more complicated, expensive perfusion circuit and the requirement of personnel for continuous monitoring of the kidney. In a recent publication, RNA sequencing of kidney tissue before and after NMP demonstrated the upregulation of genes associated with oxidative phosphorylation but also inflammatory pathways [51]. Modulation of NMP conditions by incorporating a cytokine filter into the circuit removed the inflammatory cytokines from the perfusate and reduced the inflammatory gene expression.
There is international interest in the development and clinical deployment of NMP [52] and it is the subject of other current clinical trials. The feasibility and safety of normothermic ex vivo kidney perfusion (NEVKP) trial will recruit 25 patients who receive a kidney after 1–10 h of NMP, and assess the device failure rate alongside standard measures of outcome such as DGF, graft failure and patient survival (Clinicaltrials.gov ID: NCT03136848). Perfusion at subnormothermic (20–32 °C) temperatures is also being explored [53]. A new clinical trial is due to start called ‘Oxygenated machine preservation in kidney transplantation’ (SNOPO; Clinicaltrials.gov ID: NCT04540640), which will address the safety of subnormothermic machine perfusion on transplant kidneys. This is an explorative trial that will also assess the rate of graft discard and assess graft function.
An overview of the active trials investigating both HMP and NMP is given in Table 2.
| HMP Clinical trials | ||||
| NCT Number | ||||
| Title | ||||
| Primary outcome measure | Start date | Completion Date | ||
| NCT04619732 | Real-time Monitoring of Kidney Grafts on Hypothermic Machine Perfusion | Post-operative recovery of kidney function within: 30 days of transplant | June 2021 | December 2021 |
| NCT03378817 | Hypothermic Oxygenated Machine Perfusion of Extended Criteria Kidney Allografts from Brain Death Donors | Delayed Graft Function incidence within 1 week of transplantation | December 2017 | March 2020 |
| NCT03031067 | Hypothermic Oxygenated Perfusion Versus Static Cold Storage for Marginal Graft | Graft function at 3 months post-transplantation | October 2016 | February 2018 |
| NCT04359173 | Propensity Score Matched Comparison of HMP vs. SCS in Kidney Transplantation | Delayed Graft Function incidence within 1 week of transplantation | August 2015 | March 2020 |
| NCT02055950 | Pulsed Perfusion for Marginal Kidneys |
|
July 2013 | August 2018 |
| NCT03837197 | Clinical Trial of New Hypothermic Oxygenated Perfusion System Versus Static Cold Storage | Delayed Graft Function incidence within 0–30 days of transplantation | December 2018 | December 2021 |
| NCT02876692 | Prediction and Management of Delayed Graft Function Based on Donor Criteria and LifePort Platform |
|
January 2016 | December 2019 |
| NCT02652520 | Evaluation of a Marine OXYgen Carrier: HEMO2Life for hypOthermic Kidney Graft Preservation, Before Transplantation (OXYOP) | Charting within three months of transplant:
|
March 2016 | February 2018 |
| NCT03773211 | Renaparin in Kidney Transplantation | Adverse events within 30 days | February 2019 | 1 April 2020 |
| NCT03024229 | Metabolomics in Assessing the Quality of Kidney Transplants Retained on a LifePort Perfusion Machine | Immediate graft function (IGF) ( i.e. the absence of a requirement for dialysis) within 7 days post-transplant | March 2017 | January 2020 |
| NCT01848249 | Deceased Donor Biomarkers and Recipient Outcomes |
|
May 2010 | March 2020 |
References
- NHSBT. Fact Sheet 7: Cost-Effectiveness of Transplantation. 2009. Available online: https://nhsbtmediaservices.blob.core.windows.net/organ-donation-assets/pdfs/Organ_Donation_Registry_Fact_Sheet_7_21337.pdf (accessed on 26 September 2021).
- Kaballo, M.A.; Canney, M.; O’Kelly, P.; Williams, Y.; O’Seaghdha, C.M.; Conlon, P.J. A comparative analysis of survival of patients on dialysis and after kidney transplantation. Clin. Kidney J. 2018, 11, 389–393.
- Santos, A.H., Jr.; Casey, M.J.; Wen, X.; Zendejas, I.; Rehman, S.; Womer, K.L.; Andreoni, K.A. Survival with Dialysis Versus Kidney Transplantation in Adult Hemolytic Uremic Syndrome Patients. Transplantation 2015, 99, 2608–2616.
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.; Held, P.J.; Port, F.K. Comparison of Mortality in All Patients on Dialysis, Patients on Dialysis Awaiting Transplantation, and Recipients of a First Cadaveric Transplant. N. Engl. J. Med. 1999, 341, 1725–1730.
- Yoo, K.D.; Kim, C.T.; Kim, M.H.; Noh, J.; Kim, G.; Kim, H.; An, J.N.; Park, J.Y.; Cho, H.; Kim, K.H.; et al. Superior outcomes of kidney transplantation compared with dialysis: An optimal matched analysis of a national population-based cohort study between 2005 and 2008 in Korea. Medicine 2016, 95, e4352.
- Caskey, F.; Dawnay, C.C.; Farrington, A.; Fogarty, K.; Kumwenda, F.S.; Macphee, M.; Md, S.; Steenkamp, R.; Aj, W. UK Renal Registry UK Renal Registry 18th Annual Report of the Renal Association. Nephron 2016, 132, 9–40.
- Zhong, Z.; Hu, Q.; Fu, Z.; Wang, R.; Xiong, Y.; Zhang, Y.; Liu, Z.; Wang, Y.; Ye, Q. Increased Expression of Aldehyde Dehydrogenase 2 Reduces Renal Cell Apoptosis During Ischaemia/Reperfusion Injury After Hypothermic Machine Perfusion. Artif. Organs 2016, 40, 596–603.
- Yang, Z.; Zhong, Z.; Li, M.; Xiong, Y.; Wang, Y.; Peng, G.; Ye, Q. Hypothermic machine perfusion increases A20 expression which protects renal cells against Ischaemia/reperfusion injury by suppressing inflammation, apoptosis and necroptosis. Int. J. Mol. Med. 2016, 38, 161–171.
- Chatauret, N.; Coudroy, R.; Delpech, P.O.; Vandebrouck, C.; Hosni, S.; Scepi, M.; Hauet, T. Mechanistic analysis of nonoxygenated hypothermic machine perfusion’s protection on warm ischemic kidney uncovers greater enos phosphorylation and vasodilation. Am. J. Transpl. 2014, 14, 2500–2514.
- Moers, C.; Smits, J.M.; Maathuis, M.H.J.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; van der Heide, H.; Squifflet, J.-P.; van Heurn, E.; et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2009, 360, 7–19.
- Jochmans, I.; Moers, C.; Smits, J.M.; Leuvenink, H.G.; Treckmann, J.; Paul, A.; Rahmel, A.; Squifflet, J.-P.; van Heurn, E.; Monbaliu, D.; et al. Machine Perfusion Versus Cold Storage for the Preservation of Kidneys Donated After Cardiac Death. Ann. Surg. 2010, 252, 756–764.
- Moers, C.; Pirenne, J.; Paul, A.; Ploeg, R.J. Machine Preservation Trial Study Group. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2012, 366, 770–771.
- Bradley, J.A.; Pettigrew, G.J.; Watson, C.J. Time to death after withdrawal of treatment in donation after circulatory death (DCD) donors. Curr. Opin. Organ Transpl. 2013, 18, 133–139.
- Van Erp, A.C.; Rebolledo, R.A.; Hoeksma, D.; Jespersen, N.R.; Ottens, P.J.; Nørregaard, R.; Pedersen, M.; Laustsen, C.; Burgerhof, J.G.M.; Wolters, J.C.; et al. Organ-specific responses during brain death: Increased aerobic metabolism in the liver and anaerobic metabolism with decreased perfusion in the kidneys. Sci. Rep. 2018, 8, 4405.
- Schiffer, T.A.; Gustafsson, H.; Palm, F. Kidney outer medulla mitochondria are more efficient compared with cortex mitochondria as a strategy to sustain ATP production in a suboptimal environment. Am. J. Physiol. Physiol. 2018, 315, F677–F681.
- Peris, A.; Fulceri, G.E.; Lazzeri, C.; Bonizzoli, M.; Li Marzi, V.; Serni, S.; Cirami, L.; Migliaccio, M.L. Delayed graft function and perfusion parameters of kidneys from uncontrolled donors after circulatory death. Perfusion 2021, 36, 299–304.
- Kaminski, J.; Delpech, P.O.; Kaaki-Hosni, S.; Promeyrat, X.; Hauet, T.; Hannaert, P. Oxygen Consumption by Warm Ischaemia-Injured Porcine Kidneys in Hypothermic Static and Machine Preservation. J. Surg. Res. 2019, 242, 78–86.
- Belzer, F.O.; Southard, J.H. Principles of Solid-Organ Preservation by Cold Storage. Transplantation 1988, 45, 673–676.
- Hochachka, P. Defense strategies against hypoxia and hypothermia. Science 1986, 231, 234–241.
- Bienholz, A.; Walter, B.; Pless-Petig, G.; Guberina, H.; Kribben, A.; Witzke, O.; Rauen, U. Characterization of injury in isolated rat proximal tubules during cold incubation and rewarming. PLoS ONE 2017, 12, e0180553.
- Kellerman, P.S. Exogenous adenosine triphosphate (ATP) preserves proximal tubule microfilament structure and function in vivo in a maleic acid model of ATP depletion. J. Clin. Investig. 1993, 92, 1940–1949.
- Lieberthal, W.; Menza, S.A.; Levine, J.S. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am. J. Physiol. Physiol. 1998, 274, F315–F327.
- Pefanis, A.; Ierino, F.L.; Murphy, J.M.; Cowan, P.J. Regulated necrosis in kidney Ischaemia-reperfusion injury. Kidney Int. 2019, 96, 291–301.
- Kox, J.; Moers, C.; Monbaliu, D.; Strelniece, A.; Treckmann, J.; Jochmans, I.; Leuvenink, H.; Van Heurn, E.; Pirenne, J.; Paul, A.; et al. The Benefits of Hypothermic Machine Preservation and Short Cold Ischaemia Times in Deceased Donor Kidneys. Transplantation 2018, 102, 1344–1350.
- Hendriks, K.D.W.; Brüggenwirth, I.M.A.; Maassen, H.; Gerding, A.; Bakker, B.; Porte, R.J.; Henning, R.H.; Leuvenink, H.G.D. Renal temperature reduction progressively favors mitochondrial ROS production over respiration in hypothermic kidney preservation. J. Transl. Med. 2019, 17, 1–10.
- Stubenitsky, B.M.; Ametani, M.; Danielewicz, R.; Southard, J.H.; Belzer, F.O. Regeneration of ATP in kidney slices after warm Ischaemia and hypothermic preservation. Transpl. Int. 1995, 8, 293–297.
- Chen, Y.; Shi, J.; Xia, T.C.; Xu, R.; He, X.; Xia, Y. Preservation Solutions for Kidney Transplantation: History, Advances and Mechanisms. Cell Transpl. 2019, 28, 1472.
- Hosgood, S.A.; Saeb-Parsy, K.; Wilson, C.; Callaghan, C.; Collett, D.; Nicholson, M.L. Protocol of a randomised controlled, open-label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open 2017, 7, e012237.
- Minor, T.; Horn, C.; Gallinat, A.; Kaths, M.; Kribben, A.; Treckmann, J.; Paul, A. First-in-man controlled rewarming and normothermic perfusion with cell-free solution of a kidney prior to transplantation. Am. J. Transpl. 2020, 20, 1192–1195.
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine 2020, 60, 103014.
- Nath, J.; Smith, T.B.; Patel, K.; Ebbs, S.R.; Hollis, A.; Tennant, D.A.; Ludwig, C.; Ready, A.R. Metabolic differences between cold stored and machine perfused porcine kidneys: A 1H NMR based study. Cryobiology 2017, 74, 115–120.
- Res, E.S.; Houtzager, J.H.E.; David Hemelrijk, S.; Mirza, I.C.J.H.; Idu, M.; Bemelman, F.J.; Van Gulik, T.M.; Helene, J.; Surgery, E.H. The Use of the Oxygenated AirdriveTM Machine Perfusion System in Kidney Graft Preservation: A Clinical Pilot Study. Eur. Surg. Res. 2020, 61, 153–162.
- Ravaioli, M.; Baldassarre, M.; Vasuri, F.; Pasquinelli, G.; Laggetta, M.; Valente, S.; De Pace, V.; Neri, F.; Siniscalchi, A.; Zanfi, C.; et al. Strategies to Restore Adenosine Triphosphate (ATP) Level After More than 20 Hours of Cold Ischaemia Time in Human Marginal Kidney Grafts. Ann. Transpl. 2018, 23, 34–44.
- Ravaioli, M.; De Pace, V.; Angeletti, A.; Comai, G.; Vasuri, F.; Baldassarre, M.; Maroni, L.; Odaldi, F.; Fallani, G.; Caraceni, P.; et al. Hypothermic Oxygenated New Machine Perfusion System in Liver and Kidney Transplantation of Extended Criteria Donors:First Italian Clinical Trial. Sci. Rep. 2020, 10, 1–11.
- Patel, K.; Nath, J.; Hodson, J.; Inston, N.; Ready, A. Outcomes of Donation after Circulatory Death kidneys undergoing Hypothermic Machine Perfusion following Static Cold Storage: A UK population-based cohort study. Am. J. Transpl. 2018, 18, 1408–1414.
- Bellini, M.I.; Charalampidis, S.; Herbert, P.E.; Bonatsos, V.; Crane, J.; Muthusamy, A.; Dor, F.J.M.F.; Papalois, V. Cold pulsatile machine perfusion versus static cold storage in kidney transplantation: A single centre experience. BioMed Res. Int. 2019, 2019, 7435248.
- Savoye, E.; Macher, M.A.; Videcoq, M.; Gatault, P.; Hazzan, M.; Abboud, I.; Thierry, A.; Bertrand, D.; Drouin, S.; Sayegh, J.; et al. Evaluation of outcomes in renal transplantation with hypothermic machine perfusion for the preservation of kidneys from expanded criteria donors. Clin. Transpl. 2019, 33, e13536.
- Kruszyna, T.; Richter, P. Hypothermic Machine Perfusion of Kidneys Compensates for Extended Storage Time: A Single Intervention With a Significant Impact. Transpl. Proc. 2021, 53, 1085–1090.
- Tedesco Silva, H.; Evans, R.W.; Gavaghan, M.B.; Vazquez, V.C. A Cost-Effectiveness Analysis of Organ Preservation Methods for Deceased Donor Kidneys at High Risk for Delayed Graft Function in Brazil. Transpl. Proc. 2018, 50, 3121–3127.
- Martínez Arcos, L.; Fabuel Alcañiz, J.J.; Gómez Dos Santos, V.; Burgos Revilla, F.J. Functional Results of Renal Preservation in Hypothermic Pulsatile Machine Perfusion Versus Cold Preservation: Systematic Review and Meta-Analysis of Clinical Trials. Transpl. Proc. 2018, 50, 24–32.
- Ruiz-Hernández, M.; Gómez-Dos Santos, V.; Díaz-Pérez, D.; Fernández-Alcalde, Á.; Hevia-Palacios, V.; Álvarez-Rodríguez, S.; Díez-Nicolás, V.; Elías-Triviño, S.; Burgos-Revilla, F.J. Experience with Hypothermic Machine Perfusion in Expanded Criteria Donors: Functional Outcomes. Transpl. Proc. 2019, 51, 303–306.
- Sandal, S.; Luo, X.; Massie, A.B.; Paraskevas, S.; Cantarovich, M.; Segev, D.L. Machine perfusion and long-term kidney transplant recipient outcomes across allograft risk strata. Nephrol. Dial. Transpl. 2018, 33, 1251–1259.
- Rijkse, E.; IJzermans, J.N.; Minnee, R.C. Machine perfusion in abdominal organ transplantation: Current use in the Netherlands. World J. Transpl. 2020, 10, 15–28.
- Wijk, J.; Slooff, M.J.; Rijkmans, B.G.; Kootstra, G. Successful 96- and 144-hour experimental kidney preservation: A combination of standard machine preservation and newly developed normothermic ex vivo perfusion. Cryobiology 1980, 17, 473–477.
- Hosgood, S.A.; Nicholson, M.L. First in man renal transplantation after ex vivo normothermic perfusion. Transplantation 2011, 92, 735–738.
- Hosgood, S.A.; Thompson, E.; Moore, T.; Wilson, C.H.; Nicholson, M.L. Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. Br. J. Surg. 2018, 105, 388–394.
- Hosgood, S.A.; Saeb-Parsy, K.; Hamed, M.O.; Nicholson, M.L. Successful Transplantation of Human Kidneys Deemed Untransplantable but Resuscitated by Ex Vivo Normothermic Machine Perfusion. Am. J. Transpl. 2016, 16, 3282.
- Schopp, I.; Reissberg, E.; Lüer, B.; Efferz, P.; Minor, T. Controlled Rewarming after Hypothermia: Adding a New Principle to Renal Preservation. Clin. Transl. Sci. 2015, 8, 475.
- von Horn, C.; Zlatev, H.; Kaths, M.; Andreas, P.; Minor, T. Controlled oxygenated rewarming compensates for cold storage-induced dysfunction in kidney grafts. Transplantation 2021. published online ahead of print.
- Elliott, T.R.; Nicholson, M.L.; Hosgood, S.A. Normothermic kidney perfusion: An overview of protocols and strategies. Am. J. Transpl. 2021, 21, 1382–1390.
- Ferdinand, J.R.; Hosgood, S.A.; Moore, T.; Ferro, A.; Ward, C.J.; Castro-Dopico, T.; Nicholson, M.L.; Clatworthy, M.R. Cytokine absorption during human kidney perfusion reduces delayed graft function–associated inflammatory gene signature. Am. J. Transpl. 2021, 21, 2188–2199.
- Hameed, A.M.; Miraziz, R.; Lu, D.B.; Warwick, N.; El-Ayoubi, A.; Burns, H.; Chew, Y.V.; Matthews, R.; O’Grady, G.; Yuen, L.; et al. Extra-corporeal normothermic machine perfusion of the porcine kidney: Working towards future utilization in Australasia. ANZ J. Surg. 2018, 88, E429–E434.
- Bellini, M.I.; Yiu, J.; Nozdrin, M.; Papalois, V. The Effect of Preservation Temperature on Liver, Kidney, and Pancreas Tissue ATP in Animal and Preclinical Human Models. J. Clin. Med. 2019, 8, 1421.
More
