Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Durgadevi Ravindran and Version 2 by Camila Xu.
Phytosterols, as the name implies, are defined as fatty compounds produced by plants, and remarkably contribute as the major lipid constituent of biological membrane of plant cells.
- antioxidants
- antimicrobials
- β-sitosterol
1. Introduction
Marine macroalgae (or seaweed) hold immense nutritional value and have constituted an important position in Asian diets since time immemorial. Traditionally, seaweeds viz., Undaria pinnatifida (wakame), Cladosiphono kamuranus (mozuku), Laminaria japonica (kombu) and Gelidium crinale (tengusa) are consumed as healthy foods in Japan [1]. Sargassum fusiforme, in addition to being one of the edible seaweeds consumed in Korea and China, holds an important position in traditional Chinese medicine due to its anti-atherosclerotic activity [2]. In recent times, the demands for seaweed in other parts of the world, such as North and South America, have also increased due to the migration of traditional seaweed consumers. Moreover, France has recently approved the human consumption of seaweed as condiments and vegetables, which further increases the value of seaweed in the global food market. Although seaweed has been consumed since prehistoric times, its commercial utilization in food, cosmetics, and pharmaceutical industries was recognized later [3]. Seaweed extracts are generally rich in natural growth hormones, nutrients, and trace minerals. Among several other compounds, the nutritional value of seaweed is mainly attributed to the presence of phytosterols. Phytosterols, as the name implies, are defined as fatty compounds produced by plants, and remarkably contribute as the major lipid constituent of biological membrane of plant cells. Although these plant steroids have similar chemical structures like cholesterol, the differentiation in C24 side chains makes them metabolically and functionally distinct from each other [4]. Like terrestrial plants, seaweeds as well, exhibit the diversified composition of phytosterol contents with a similar profile of health benefits and their prevalence in this phytoplankton is largely influenced by their evolutionary origin [5][6][5,6]. For instance, brown algae (phaeophyta) predominantly contain phytosterols such as fucosterol and brassicasterol, with a small proportion of plant cholesterol and therefore considered a promising source for phytosterols. In contrast, red algae (rhodophyta) contain cholesterol as their principal sterol content, with a minor quantity of phytosterols such as sitosterol, fucosterol, chalinasterol and desmosterol. On the other hand, green algae (chlorophyta) vary in their types of sterols, such as ergosterol, chondrillasterol, β-sitosterol, 28-isofucosterol, cholesterol and poriferasterol, depending on the species [7][8][7,8]. Furthermore, to date, there have been no studies found to demonstrate the obvious negative effects (in terms of toxicity) of phytosterols on humans. Accordingly, international agencies such as the Food and Drug Administration (FDA) and the European Union Scientific Committee (EUSC) have already approved phytosterols as safe to use [9].
2. Biosynthesis of Phytosterols
The sterol biosynthesis pathway is an important pathway of living organisms, exhibited by certain bacteria and all eukaryotes [10][11][10,11]. Recent discoveries have increased the complexity of this pathway, urging researchers to decipher it further in order to deepen perceptions of its activity. Phytosterol biosynthesis is a branch of sterol synthesis found in almost all plant species and it can be distinguished from the sterol biosynthesis pathway of all eukaryotic kingdoms, such as animals and fungi. Although seaweeds are known to be potential producers of phytosterols, not all species of seaweeds have evolved to do so. Only limited species, such as brown seaweed, have the ability to produce phytosterols such as fucosterol and saringosterol. These sterols have therapeutic implications, such as neurostimulatory effects, and thus have piqued the interest of the clinical community [12][13][12,13]. The paucity of investigation and the diversity of macroalgal species have obscured the identification of pathways responsible for phytosterol synthesis. Nevertheless, Calegario et al. [14] postulated that seaweeds may use the traditional pathways of plants for isoprene unit synthesis. As in plant species, the phytosterol biosynthesis in macroalgae can be stratified into three major segments: (i) the biosynthesis of isoprene, (ii) the condensation of isoprene into triterpenoids and their epoxidation and (iii) the biosynthesis of phytosterols [15]. In these sequential reactions, products of glycolysis are converted into hydrocarbons such as isoprene to triterpenoids and final end products of phytosterols [16].
2.1. Biosynthesis of Isoprene
Isoprene is the basic unit of all isoprenoids and terpenoids with a five-carbon functional group [17]. In a eukaryotic cell, the synthesis of isoprenes, namely isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) can be carried out through two unique pathways called mevalonate (MVA) and methylerythritol phosphate (MEP) pathways. All plant species can utilize both these pathways to generate the precursors of isoprenes. In contrast, green macroalgae are found to use the MEP pathway, as they lack the genes involved in the MVA pathway. However, red algae have the potency to synthesize IPP and DMAPP through both these cytosolic- and plastid-relying mechanisms. It is postulated that during endosymbiosis, green algae could have lost machinery from the MVA pathway and evolved with most relying on the MEP pathway for all fundamental aspects of isoprenoid synthesis. Conversely, primary endosymbionts, such as plants and red algae, have retained both MVA and MEP pathways over their evolutions [18]. Most portions of the MVA and MEP pathways transpire in the cytoplasm and plastid, respectively. Interestingly, all the sequential events of the MEP pathway ensue in plastids; however, the genes involved in these processes are encoded by the nuclear genome [19][20][19,20]. Furthermore, the MEP pathway produces both IPP and DMAPP directly, whereas MVA produces only IPP that is followed by isomerization of IPP into DMAPP by IPP isomerase (IPPI). As green algae rely solely on the MEP pathway for isoprenoid synthesis, they have evolved to express chloroplast membranal antiporters to maintain a balanced flux of IPP/DMAPP between plastid and cytosol [21]. Though the MEP pathway produces both these precursors, the ratio of IPP: DMAPP generated through this event varies among plant species.
The MVA pathway comprises six enzymatic reactions, which commence with acetyl-CoA condensation by acetyl-coA C-acetyltransferase (ACCT), followed by synthesis of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) by HMG-CoA synthase (HMGS) with subsequent reduction by HMG reductase (HMGR) to form MVA as an intermediate. MVA is then pyrophosphorylated and decarboxylated in subsequent steps by mevalonic acid (MK), phosphomevalonate (PMK) kinases and mevalonate-5-diphosphate decarboxylase (MPDC), respectively, to form the final isoprene unit, IPP. In the MEP pathway, the final product pyruvate, and intermediate- glyceraldehyde-3-phosphate (G3P), of glycolysis are converted into isoprene via seven successive steps. Pyruvate and G3P are condensed together to produce 1-deoxy-D-xylulose 5-phosphate (DXP) by DXP synthase (DXS), followed by reduction, cytidylation, phosphorylation, decytidylation and final reduction by DXP-reductoisomerase (DXR), 2-C-methyl-derythritol 4-phosphate cytidylyltransferase (MCT), 4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol kinase (CMK), 2-C-methyl- D-erythritol 2,4-cyclodiphosphate synthase (MDS), 4-hydroxy-3-methylbut-2-enyldiphosphate (HMBPP) synthase (HDS) and HMBPP reductase (HDR), respectively, to generate the end isoprene products IPP and DMAPP (Figure 1A).
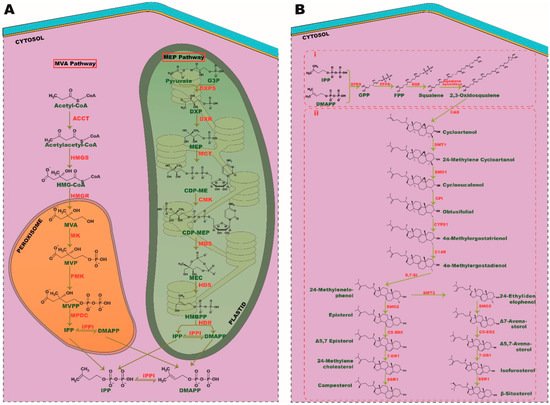

Figure 1. Biosynthesis pathways of phytosterols. (A) Isoprene unit, IPP, and DMAPP synthesis. A product of glucose metabolism, acetyl-coA and pyruvate is converted into isoprene units through MVA and MEP pathway, respectively. (B) (i) Condensation of isoprene into triterpenoids and (ii) phytosterol synthesis. SMT, sterol C-24 methyl transferase; SMO, sterol C-4 methyl oxidase; CPI, cyclopropyl sterol isomerase; CYP51, sterol C-14 demethylase; C-14R, sterol C-14 reductase; 8, 7-SI, sterol 8, 7 isomerase; C5-SD2, sterol C5(6) desaturase; 7DR1, 7-dehydrocholesterol, SSR1, sterol side-chain reductase 1.
2.2. Condensation of Isoprene into Triterpenoids and Their Epoxidation
Isoprene, with a five-carbon atom, condenses together to generate monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), and triterpenes (C30). Triterpenes serve as precursors for phytosterol synthesis. Isoprene from the MEP pathway catalyzes the synthesis of monoterpenes, diterpenes, chlorophyll, carotenoids, and other phytohormones, such as gibberellin, strigolactone, and abscisic acid. It is indeed a case that the MVA pathway-derived isoprene is responsible for the synthesis of the phytosterol precursor, squalene (a triterpene), via geranyl pyrophosphate (GPP) and farnesyl pyrophosphate (FPP), which are the precursors of monoterpenes and triterpenes, respectively [22][23][22,23]. FPP synthase (FPPS) and squalene synthase (SQS) catalyze these sequence reactions, which follow the epoxidation of squalene into 2, 3-oxidosqualene by squalene epoxidase (Figure 1Bi).
2.3. Phytosterol Synthesis
The biosynthesis of phytosterol exhibits a parallel pathway of cholesterol synthesis in eukaryotic cells [24]; 2, 3-oxidosqualene serves as a common precursor for all sterol synthesis i.e., sterols like cholesterol in humans, ergosterol in fungi and phytosterols in plants. The biosynthesis of cholesterol and ergosterol is catalyzed by lanosterol synthase (LAS), whereas phytosterol synthesis from oxidosqualene is catalyzed by cycloartenol synthase (CAS) [25]. As in plant species, seaweeds metabolize 2, 3-oxidosqualene to phytosterol by CAS and exhibit the hybrid cholesterol pathway as well, indicating an evolutionary relationship between these two phototrophic organisms [14].
Numerous studies have highlighted the importance of the CAS gene in phytosterol biosynthesis. In extensive research by feeding experiments using [6-13C2H3] MVL, Ohyama et al. [26] demonstrated that a mere 1.5% of sitosterol, encoded by the LAS1 gene, emphasizes its crucial role in the biosynthesis of phytosterols. Cycloartenol is metabolized into 24-methylenelophenol through six enzymatic reactions, such as methylation, double demethylation, double isomerization, and a reduction. From 24-methylenelophenol, the pathway branches off into two separate pathways, giving rise to episterol and Δ7 avenasterol via 24-ethylidenelophenol and end up in campesterol and β-sitosterol, respectively. Stigmasterol is synthesized directly from -sitosterol, whereas campesterol is converted into brassinolide through subsequent reactions [24] (Figure 1Bii).
3. Extraction and Characterization of Phytosterols
Extraction techniques are proficient in separating the soluble metabolites of seaweed using suitable solvents [27]. The isolation techniques to be used for the extraction of phytosterols generally depend on the type of phytosterols (free, glycosylated, and esterified) and the nature of the matrix [28]. In each technique, optimized experimental conditions and adequate parameters are required to attain the appropriate quantity and higher yields from seaweed extract. The quality of an extract is substantially influenced by various factors, such as plant/seaweed material, solvent, extraction procedure, and others [29]. There are two common extraction techniques, namely conventional and non-conventional extraction (Figure 2) [30]. Conventional techniques usually employ a large volume of organic solvents to extract adequate analytes from samples that are required for further analysis. Nonetheless, the utilization of a higher volume of organic solvents may have a negative impact on human health and the environment. Furthermore, conventional techniques are reported to possess several limitations, such as the necessity of solvents with very high purity, an extended period of time for extraction, low selectivity of extraction, requirement of solvent evaporation and thermal decomposition of heat liable compounds. These limitations demanded the discovery of new extraction techniques with relatively very low utilization of organic solvents and more advantages than conventional techniques [31].
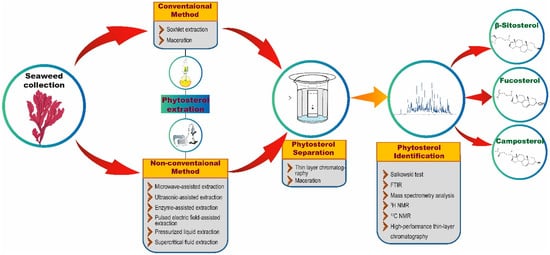

Figure 2.
An overview of phytosterol extraction and characterization methods.
Soxhlet extraction is one of the conventional techniques, and remains the most preferred extraction technique for phytosterols, still, today [32][33][32,33]; it serves as a standard to many newly developed extraction techniques (Table 1) [31]. For example, Poulose et al. [32] extracted phytosterols from the red seaweed Gelidium spinosum using the Soxhlet method and revealed the presence of stigmasterol, with a mass of 412.69 g/mol, through Fourier transform infrared (FTIR) and gas chromatography–mass spectrometry (GC–MS). In addition, maceration is another common conventional technique, which is stated to be simple and cost-effective for phytosterol extraction [34].
Table 1.
Examples of phytosterols identified from seaweeds.
| Source | Extraction Method | Methods of Analysis | Identified Phytosterols | References | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gelidium spinosum | Soxhlet method | FTIR and GC–MS | Stigmasterol | [32] | |||||||||||||||||||||||||||||||||
| Saccharina latissima | saponified extract | GC–MS | cholesterol, desmosterol, 24-methylenecholesterol, fucosterol, cycloartenol, and unknown ∆ | 5 | -sterol | [35] | |||||||||||||||||||||||||||||||
| Palmaria decipiens, Plocamium cartilagineum, Iridaea cordata, and Pyropia endiviifolia | alkaline hydrolysis | GC–MS | cholesterol, brassicasterol, campesterol, stigmasterol, β-sitosterol, and fucosterol | [33] | |||||||||||||||||||||||||||||||||
| Ecklonia radiata | alkaline saponification | LC-MS/MS and GC–MS | fucosterol, Sitostanol, 24α-methyl cholesterol, and 24α-ethyl cholesterol | [36] | |||||||||||||||||||||||||||||||||
| Padina australis | and | Stoechospermum marginatum | , and | Ahnfeltiopsis pygmaea | acid and alkaline hydrolysis followed by solvent extraction, derivatization, and GC determination | gas chromatography coupled with a flame ionization detection system (GC–FID) | sitostanol, campestanol ergosterol, campesterol, delta-5-avenasterol, stigmasterol, sistenol, cholesterol, and 24-methylenecholesterol | [37] | |||||||||||||||||||||||||||||
| Adenocystis utricularis | , | Desmarestia confervoides | , | Curdiea racovitzae | , | Myriogramme manginii | , and | Ulva intestinalis | Soxhlet method | GC–MS and FT-IR | fucosterol, cholesterol, and hydroxymethylcholesterol | [38] | |||||||||||||||||||||||||
| Phaeophyta ( | Cystoseira barbata | , | Cystoseira compressa | , | Fucus virsoides | ) and chlorophyta ( | Codium bursa) | agitation-assisted extraction and pressurized liquid extraction | TLC | cholesterol, brassicasterol, campesterol, campestanol, stigmasterol, β-sitosterol, fucosterol, and isofucosterol | [39] | ||||||||||||||||||||||||||
| Cystoseira trinodis | solvent extraction and column chromatography | 1 | H, | 13 | C NMR, heteronuclear multiple-bond correlation (HMBC), heteronuclear single-quantum coherence (HSQC), GC–MS, and electron ionization-mass spectra (EI-MS) | saringosterol, β-sitosterol | [40] | ||||||||||||||||||||||||||||||
| Sargassum horneri | high-speed countercurrent chromatography | NMR | fucosterol and saringosterol | [41] | |||||||||||||||||||||||||||||||||
| S. fusiforme | Folch method | GC–MS | 24(S)-Saringosterol | [42] | |||||||||||||||||||||||||||||||||
| Halimeda tuna, Codium bursa, and Cystoseira barbata | solvent extraction | GC and GC–MS | fucosterol, campesterol and β-sitosterol | [43] | |||||||||||||||||||||||||||||||||
| Sargassum elegans | solvent extraction and column chromatography | NMR ( | 1 | H and | 13 | C), IR and mass spectral data | β-sitosterol, fucosterol | [44] | |||||||||||||||||||||||||||||
| Phaeophyta ( | Cystophora pectinata | , | Pyllospora comoasa | , | Scytothalia dorycarpa | , | Carpoglossum confluens | , | E. radiata | , | Sargassum lacerifolium | , | Perithalia caudata | , | Codium harveyi | , | Scytothalia dorycarpa | , | Hypnea valida | , | Cystophora monilifera | , | Hormosira banksia | , | Myriodesma integrifolium | , Epiphytic algae sp., | Cystophora subfarcinata | ), Rhodophyta ( | Austrophyllis harveyana | , | Rhodophyllis membaneacea | ), and chlorophyta | (Codium fragile) | maceration | post-chromatographic derivatization and HPTLC | β-sitosterol | [45] |
| Ascoseira mirabilis | , | A. utricularis | , | Desmarestia anceps | , and | Phaeurus antarcticus | saponification | GC–MS | cholesterol, desmosterol, brassicasterol, campesterol, stigmasterol, fucosterol, and β-sitosterol | [46] | |||||||||||||||||||||||||||
| Ecklonia stolonifera | silica gel column chromatography | 1 | H and | 13 | C NMR | fucosterol | [47] | ||||||||||||||||||||||||||||||
| Rhodophyta ( | Gracilaria vermiculophylla | , | Pterocladiella tenuis | , | Palisada intermedia | , | Chrysymenia wrightii | , | Gracilaria elegans | , | Grateloupia asiatica | , | Laurencia okamurae | ) and Phaeophyta ( | Eckloniopsi radicosa | , | Sargassum thunbergia | , | Ecklonia kurome | , | Eisenia arborea | , | Sargassum piluliferum | , | S. fusiforme | , | U. pinnatifida | , | Ecklonia cava | ) | saponification | HPLC with fluorescence detection | cholesterol, β-sitosterol, ergosterol, stigmasterol, and fucosterol | [48] | |||
| S. horneri | total lipid extraction using methanol | RP-HPLC | fucosterol | [49] | |||||||||||||||||||||||||||||||||
| Hizikia fusiformein | ethanol extraction and chromatographic separation | LC/ electrospray ionization (ESI)-MS | fucosterol | [50] | |||||||||||||||||||||||||||||||||
| A. utricularis | , | Ascoseira mirabilis | , | Cystosphaera jacquinotii | , | D. anceps | , | Durvillaea antarctica | , and | Himantothallus grandifolius | ultrasound irradiation | LC-MS/MS | ergosterol, brassicasterol, fucosterol, β-sitosterol, campesterol, cholesterol, and stigmasterol) | [6] | |||||||||||||||||||||||
| Porphyra dentata | methanol extraction and silica gel column chromatography | HPLC- evaporative light scattering detector (ELSD) | cholesterol, β -sitosterol, and campesterol | [51] |
Apart from these conventional techniques, phytosterol extraction has also been shown to be accomplished using many other non-conventional extraction techniques, such as microwave-assisted extraction (MAE), ultrasonic-assisted extraction (UAE), enzyme-assisted extraction (EAE), pulsed electric field-assisted extraction (PEFAE), pressurized liquid extraction (PLE), and supercritical fluid extraction (SFE) (Figure 2) [31]. For instance, Xiao et al. [52] have employed MAE with high-speed countercurrent chromatography (liquid-liquid partition chromatography) and a UV detector to extract, separate, and purify phytosterols from the edible brown seaweeds S. fusiforme and U. pinnatifida. By employing these extraction and chromatographic techniques, the authors were able to obtain 1.5 mg of 24-methylenecholesterol and 13 mg of fucosterol from 15 g of U. pinnatifida and 0.3 mg of 24-methylenecholesterol and 4.6 mg of fucosterol from 15 g of S. fusiforme. Remarkably, Roiaini et al. [53] have performed a comparative analysis of various phytosterol extraction techniques on cocoa butter such as Soxhlet, ultrasonic, supercritical carbon dioxide, and supercritical carbon dioxide with co-solvents. The authors concluded that the highest phytosterol content was obtained when using supercritical carbon dioxide with a cosolvent.
The separation process usually follows the extraction process. In this context, thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC) techniques are extensively used separation techniques (Figure 2). After the separation process, phytosterols in the analytes are confirmed via various analytical methods. Preliminary screening of phytosterols can be performed through Salkowski test [54], in which the formation of brown ring confirms the presence of phytosterols. Besides the conventional Salkowski method, FTIR and nuclear magnetic resonance are widely utilized to analyze phytosterols. However, precise determination of phytosterols could be achieved through employing mass spectrometry analysis [32][33][32,33]. For example, GC–MS analysis of the ethanolic extracts of 18 seaweeds revealed the presence of 14 compounds including 3 sterols, cholestanol, β-sitosterol, and fucosterol [55]. In another study, the presence of a significant amount of fucosterol in brown seaweed C. barbata and the highest level of β-sitosterol in H. tuna and C. bursa were revealed through GC–MS analysis [43]. Similarly, Santi et al. [33] have isolated sterols from four red seaweeds namely P. decipiens, P. endiviifolia, I. cordata, and P. cartilagineum using alkaline hydrolysis extraction method and showed the presence of sterols such as fucosterol, β-sitosterol, stigmasterol, brassicasterol, cholesterol and campesterol through GC–MS analysis. Correspondingly, Kendel et al. [56] have also employed GC–MS analysis to reveal the presence of phytosterols such as fucosterol, isofucosterol, brassicasterol, chondrillasterol and cholest-4-en-3-one in the chloroform/methanol extracts of U. armoricana and S. chordalis.
In another study, Bouzidi et al. [57] used 1H nuclear magnetic resonance (NMR) to analyze fractions of the marine brown seaweed C. foeniculacea that were extracted using a chloroform methanol water mixture and isolated using reversed-phase HPLC (RP-HPLC). Through 1H NMR analysis, the authors revealed the presence of fucosterol and an epimeric combination of saringosterol in the seaweed fractions. Recently, a one-step preparative method for separation of phytosterols was put forward by Xia et al. [41] wherein the authors successfully utilized the high-speed countercurrent chromatographic method to extract fucosterol (23.7 mg), and saringosterol (3.1 mg) from the crude extract of S. horneri using two-phase solvent system. Furthermore, the authors have characterized the phytosterols through 1H and 13C NMR structural analysis. In addition, high-performance thin-layer chromatography (HPTLC, a technique for target-directed identification of active leads in a group of compounds) combined with biochemical and microchemical derivatizations revealed the presence of phytosterols and phenolic lipids in the ethyl acetate extract of 19 marine algae samples in addition to displaying bioactivity such as antioxidant, α-amylase and acetylcholinesterase inhibitory activities [41]. In a study by Oh et al. [47], methanolic extract of E. stolonifera was fractionated using various solvents and the fraction with strong bioactivity was further purified to yield fucosterol (99% purity, determined by HPLC) and identified by 1H and 13C NMR methods to study the neuroprotective effects of fucosterols.
Through various techniques, the sterol profiling of diverse seaweeds from different regions has already been done by many research groups, which has subsequently helped to gratify the demand for phytosterols in the global market. By uncovering the concentration of diverse sterols in different seaweeds of different regions during various seasons, the optimal techniques and suitable seaweeds could be easily identified for the preparation of particular phytosterols in large quantities. Furthermore, the extraction, isolation and analytical techniques are continually evolving, which simplifies the overall preparation process of phytosterols eventually and also proves to be more competent, fast and eco-friendly than conventional techniques.
