You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by SEYED YAZDAN MADANI and Version 2 by Rita Xu.
Cancer is a disease that has resulted in millions of deaths worldwide. The current conventional therapies utilized for the treatment of cancer have detrimental side effects. This led scientific researchers to explore new therapeutic avenues with an improved benefit to risk profile.
- magnetic nanoparticles
- cancer
- synthesis
- functionalization
1. Introduction
Cancer is the biggest killer worldwide, with an estimate of 19.3 million new cancer cases and almost 10 million cancer death occurring in 2020 [1]. In the United Kingdom alone, there are over 510 deaths daily. This has devastating effects on the family, especially children, as well as all to society and economics. Deaths from cancer across the world are expected to rise, with an estimation of 12 million deaths by 2030 [2]. Although considerable progress has been done in the treatment of cancer over the last 50 years, it remains a huge health concern [3]. Consequently, the advancement of effective resources for the diagnosis, monitoring, and treatment of cancer is a continuous challenge. Some of the current therapies used for cancer treatment are radiation therapy, chemotherapy, and surgery [4]. Whilst these have been the conventional therapies used for decades, they do have their disadvantages and side effects. For example, the surgical removal of tumours is mostly limited to large, accessible, and resectable tumours. Chemotherapeutic agents only target cells that are rapidly dividing, which means they will not only kill cancer cells, but normal cells such as bone marrow cells as well [5]. Chemotherapy can result in serious side effects such as hair loss, nerve damage, nausea, and infertility [6]. Radiation therapy such as gamma rays unavoidably causes healthy tissues to deteriorate along the path of radiation. In consideration of the limitations of current treatments, it is crucial to improve cancer therapies to specifically target tumour cells and avoid healthy tissues [7]. For this reason, an enormous effort has been dedicated to researching new therapeutic approaches [3]. The application of nanomaterials for treatment of cancer is emerging as a possible viable option and has entered the phase of clinical application [8].
Nanotechnology is a field that has drawn the attention of scientific communities around the globe. The notion of nanotechnology was presented in a lecture by Nobel Laureate Richard Feynman at the California Institute of Technology, in December of 1959 [9]. In the subsequent years, various aspects of nanomaterials have been researched. This involves the engineering of these minute nanostructures, in the context of their surface chemistries, chemical composition, binding ligands, antibodies for certain activities, and lowering of toxicity levels [10]. The formation of nanomaterials is largely dependent on the cross-collaboration of several disciplines throughout science to modify and transform the atomic dimensions of materials [11].
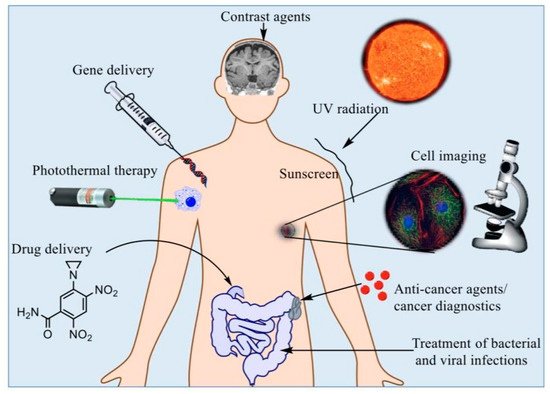
The development of the field in the form of various nanostructures, such as quantum dots, metallic nanoparticles, fullerenes, and magnetic nanoparticles (MNPs), has attracted a considerable amount of attention from the electronic, material, and medical sciences [9]. This field has gained popularity and competitive demand owing to its subsequent social and economic impacts [12]. The relationship between nanotechnology, biology, and medicine is a fast-moving and interesting area of research. Multiple experts have indicated that the use of nanotechnology in medicine, also known as “nanomedicine”, provides numerous exciting opportunities for health care in the future and could transform the areas of tissue engineering, targeted drug delivery, and disease detection [13][14][15][16][13,14,15,16]. Early detection of diseases is very desirable in order for better health outcomes and lessening social-economic strains [17][18][19][17,18,19]. In particular, quick and sensitive identification of disease biomarkers will instantaneously become valuable for diagnostic screening. It will also facilitate the monitoring of disease progression and the efficiency of treatment [20][21][20,21]. Nanotechnology platforms have shown to be exceptional agents for biomedical applications (Figure 1) [22].
Nanoparticles is a term that refers to materials with one dimension at a minimum, ranging between roughly 1 and 100 nanometres (nm), normally containing a couple of hundreds to 105 atoms [24]. Nanoparticles are composed of organic (e.g., polymeric) or inorganic materials which could be biodegradable. Their significance stems from the fact that the properties of nanoparticles differ from those of bulk materials formed from the same configuration. This is largely due to size effects and the role of surface phenomena with size reduction. Nanoparticles can either act at the cellular or tissular level. When acting on the cellular level, the nanoparticles can be endocytosed or phagocytosed (for example, by macrophages or dendritic cells), leading to internalization. Through this approach, the nanoparticles may transcend beyond the cytoplasmic membrane, and in some instances, beyond the nuclear membrane (for example, transfection applications) [25]. In recent times, nanoscale materials were the main focus of research, specifically in the areas of tissue engineering and regenerative medicine. A few examples include nanofibers, nanotubes, and nanoparticles, which can all be specifically adjusted to their function and purpose in tissue engineering [26]. The main advantage of utilizing nanoparticles is that they can be precisely manipulated and directed to a certain biological marker or entity and engage on a protein (3–50 nm), genetic (10–100 nm), subcellular (20–250 nm), or cellular level (10–100 nm) [27][28][27,28]. Their distinct dimensions, along with their special characteristics, have increased their attraction within this field [29]. Nanoparticles are found in nature, but can also be a consequence of human activity. They have distinctive qualities owing to their submicron size. This includes a larger surface area to volume ratio, a great value of fraction of near-surface layers and surface atoms, and the capability to display quantum effects. Their uncommon characteristics cannot be predicted based on the features of bulk materials. They are widely used in a number of technical and scientific areas, including catalysis, ecology, engineering, and healthcare [30].
MNPs are one of the most extensively researched nanomaterials, owing to their potential uses in various areas of research [31]. MNPs have already been used for the detection of cancer by localising the sentinel node, as well as molecular imaging [32]. MNPs are extensively being researched for usage in various industrial and scientific areas, varying from mass data storage to catalysis. The concept of using MNPs to target tumour cells within the human body for the treatment of cancer was first suggested late in the 1970s [33]. MNPs can be used to heat up the tumour and kill it, which is due to tumours cells being more sensitive to temperature increase than healthy ones. By getting the MNPs into the tumours, then releasing energy as heat when subjected to an alternating magnetic field, this will damage the cancer cells [34]. Those magnetic nanomaterials are categorised into five main types: ferromagnetic (such as cobalt, nickel, and iron), paramagnetic (such as magnesium, lithium, tantalum, and gadolinium), diamagnetic (such as silver, copper, gold, and the majority of known elements), antiferromagnetic (such as CoO, MnO, CuCl2, and NiO), and ferrimagnetic (such as maghemite γ-Fe2O3 and magnetite Fe3O4) [24]. The chemical and physical characteristics of MNPs are significantly reliant on their size, shape, crystalline structures, and chemical components. Additionally, MNPs have special magnetic properties such as low Curie temperature, superparamagnetism, and great magnetic susceptibility [35]. Magnetic susceptibility is the ratio of magnetization to an applied field that demonstrates how strongly a nanoparticle is either attracted to or repelled by a magnetic field [36].
In regard to MNPs, the fundamental idea was to affix conventional anticancer drugs to small magnetic spheres externally, prior to administering them into the human body. Once injected into the blood stream, strong external magnetic fields would gather the drug loaded nanoparticles within the tumour tissue. It is expected that with this method, the drug payload will be considerably decreased. Therefore, the undesirable side effects linked to the systematic distribution of chemotherapeutics, including hair loss, nausea, and a weakened immune system, would be prevented. Despite the fact that it is still not fully in clinical use, nanomedicine has made great strides from these original concepts and is progressing at a phenomenal speed [33].
MNPs ranging from 10 to 100 nm are favourable for use in-vivo, considering they do not present with rapid renal clearance, as nanoparticles smaller than 10 nm do, and do not become internalised by the reticuloendothelial system, as nanoparticles over 200 nm do [37]. All MNPs utilized in-vivo thus far are comprised of the iron oxides maghemite (γ-Fe2O3) and magnetite (Fe3O4), owing to their recognized pathways of metabolism and their low levels of toxicity. Both oxides’ crystal structures are based upon a cubic dense packing of oxygen atoms, only varying in their distribution of iron ions inside the crystal lattice [38]. Iron oxide MNPs are the most favourable nanomaterials in medical sciences as a result of their characteristics of biocompatibility, stability in aqueous solutions, low toxicity, and brilliant physiochemical properties such as superparamagnetism [9]. The widespread use of iron oxide MNPs is also attributed to their ability to manipulate particle motion, cause energy dissipation, and provide imaging contrast upon exposure to an external magnetic field [39]. The magnetic response of iron oxide is stable due to its low sensitivity to oxidation [40]. Moreover, iron oxide MNPs have an advantage over alternative metal nanoparticles because of their size control, specific interaction and dispersion, and avoidance of aggregation by coating and penetration of cell and tissue barriers [9]. Overall, MNPs acquired greater attention because of their unique behavioural, structural (Figure 2), and diversified applicable qualities. For instance, their distinct magnetic properties and adjustable size, functionalizable surface with various molecules, biocompatibility with different cell types, high chemical stability with increased surface area, inductive magnetic moment, high magnetic susceptibility and superparamagnetism [22]. Nanoparticles that are superparamagnetic and biocompatible are immediately injected into tumour tissue, where they can be controlled by an external magnetic field to generate heat as a consequence of the Brownian and Néel relaxation processes [38].
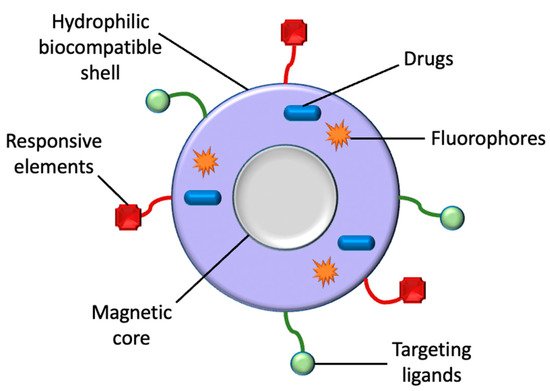
Figure 2. A classic illustration of a magnetic nanoparticle’s structure. Aside from drugs, MNPs can also be utilized for the transportation of targeting ligands, fluorophores, and responsive elements for their respective biomedical applications.
In the interest of further expanding the opportunities these MNPs can offer, researchers have attempted to adjust their magnetic properties through modifying their size, shape, and morphology. It should be noted that in recent times MNPs with an internal cavity have been invented, creating hollow structures. From the perspective of magnetism, this “hollow” structure is particularly fascinating because the existence of both inner and outer surfaces leads to MNPs with enhanced total surface areas. This results in increased surface disorder and thus higher surface anisotropy and exchange bias. Recently, various types of hollow MNPs with improved qualities have been announced in the literature. A majority of them are based on magnetic oxides and ferrites. Hollow MNPs with adjustable shell thickness and composition are excellent constituents for new enhanced materials that can be used for a lot of possible applications. In theory, hollow structures allow the encapsulation of various contents inside the MNPs for different applications. For instance, the hollow MNPs may be encapsulated with anticancer drugs and used for drug delivery applications [41]. In comparison to normal nanoparticles, hollow nanoparticles take advantage of the greater surface area and pore volume, as well as the additional paramagnetic centres, meaning that they could yield an enhanced longitudinal relaxation performance [42]. Essentially, metal nanoparticles exemplify an important gateway for the future of medicine [23].
A number of approaches were suggested for the synthesis of MNPs, i.e., reverse micelles synthesis, co-precipitation, hydrothermal synthesis, and thermal reduction or decomposition [9]. Thus far, methods based upon alternative mechanisms, such as the nanoscale Kirkendall effect, were highly developed to synthesise several hollow nanoparticles, for instance hollow iron oxide nanoparticles [42]. Following the synthesis of MNPs, various surfactants and polymer coatings were utilized, for example dextran and polyethylene glycol (PEG) [9]. The functionalization of MNPs is desirable, as it will improve their biocompatibility, protect their magnetic core from oxidation, and prevent the nanoparticles from agglomerating.
In the last 20 years, MNPs have been applied in various biomedical applications such as drug delivery, cancer therapy, and magnetic resonance imaging (MRI) [43]. In addition, hollow MNPs have several biomedical applications of their own. This involves the simultaneous provision of diagnosis and therapy, since the large cavity found within the hollow nanostructure could be utilized to contain different biomolecules and drugs that are released in a regulated manner. Furthermore, the surface of hollow MNPs could be functionalised with targeting agents [44].
Although there have been many developments in the area of nanomedicine in recent years, there is still a lot of drawbacks. For instance, the long-term toxicity of several nanoparticles is not widely known owing to the newness of this field of research [23]. Additionally, despite it not entirely being in clinical use yet, nanomedicine has made great progress and is advancing at extraordinary speed [45].
2. Applications of Magnetic Nanoparticles for Diagnostic and Treatment of Cancer
MNPs are an optimal choice for drug delivery owing to their low toxicity levels, great targeting efficiency, and large surface area to volume ratio [46][85]. Additionally, MNPs can be utilized in magnetic hyperthermia to destroy cancer cells and decrease tumour volume via a targeted approach [47][86]. Moreover, the magnetic qualities of MNPs can be employed for significant imaging modalities, such as MRI [22]. The imaging qualities of MNPs make them an ideal option for the concurrent provision of diagnosis and therapy, also known as theranostics [47][86]. MNPs offer a greater theranostic capacity in comparison to liposomes or alternative polymer-based nanoparticles, due to their magnetic properties [48][87]. MNPs are beneficial in theranostics as a result of their ability to concurrently be directed, visualized, and heated by external magnetic fields [43]. This section will discuss the application of MNPs in drug delivery, magnetic hyperthermia, and diagnosis (MNPs applied as MRI contrast agents), since they are the most widespread applications of MNPs at present (Figure 3).
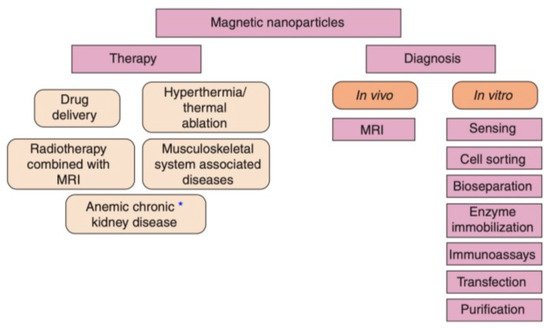

Figure 3. The range of available biomedical applications for MNPs. The asterisk (*) represents either diagnostic or therapeutic applications that are undergoing clinical trials [25].
3. Application of Magnetic Nanoparticles in Drug Delivery
The great extent of non-specificity associated with the utilization of drugs is a significant disadvantage. Following oral or intravenous administration, the systemic distribution of drugs leads to the development of adverse effects, and less of the active drug reaches the target site. Thus, larger doses are required when administering conventional anti-cancer drugs in order to achieve an appropriate local concentration at the site of action. This is a major problem, particularly for anti-cancer drugs that demonstrate severe adverse effects, such as cardiomyopathy, neurotoxicity, hair loss, and bone marrow suppression [49][88].
Multiple strategies relating to the delivery of drugs to tumour regions were considered. In 1960, it was suggested that MNPs could be transported via the circulatory system to a specific site within the body, with the guidance of a magnetic field. After the 1970s, advancements in the application of MNPs for the delivery of chemotherapeutic agents was observed [25].
Nanoparticles utilized in drug delivery can simultaneously enhance drug stability, and overcome the issues associated with the administration of conventional anti-cancer therapies. For example, superparamagnetic iron oxide nanoparticles (SPIONs) functionalized with PEG and conjugated with doxorubicin (SPIO-PEG-D) were developed for chemotherapy. By conjugating doxorubicin onto the surface of SPIONs with PEG, the half-life of doxorubicin was extended. An in-vitro experiment demonstrated that SPIO-PEG-D results in reduced DNA expression and increased cell apoptosis for HT-29 cancer cells. Additionally, in-vivo experiments illustrated the reduction of cardiotoxic and hepatotoxic side effects due to the combination of this drug delivery system and an external magnetic field [50][89].
MNPs achieve controlled and specific drug release by attaching to drug molecules via a cleavable linker, or a polymeric shell created with the ability of releasing drugs. Those MNPs can be guided to a specific site by the application of an external magnetic field, and the drugs can be delivered as a result of either enzymatic cleavage or modifications in the physiological environment, such as temperature or pH [43]. For example, nanoparticles were synthesized with methotrexate and dendrimer- doxorubicin attached onto their surface via amide or cleavable hydrazone bonds [51][90]. Therefore, when the nanoparticles are taken up into the cells, the bonds cleave owing to the presence of lysozymes. Through passive targeting (Figure 4), the nanoparticles improved the delivery of doxorubicin to the tumour site, and drug release only took place at lysosomial pH [43].
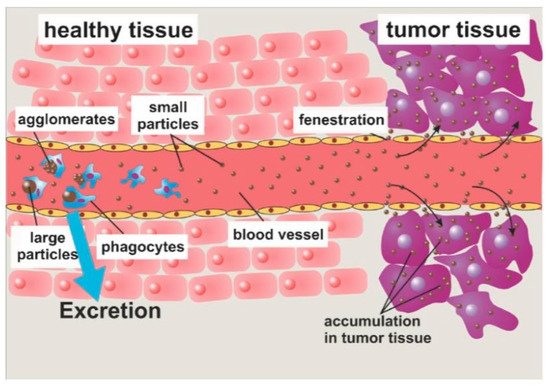

Figure 4. Passive targeting occurs as a result of the enhanced permeability and retention (EPR) effect. Large particles are engulfed by phagocytes, whilst small particles proceed to the target. The EPR effect allows the MNPs to accumulate within the target tumour [33].
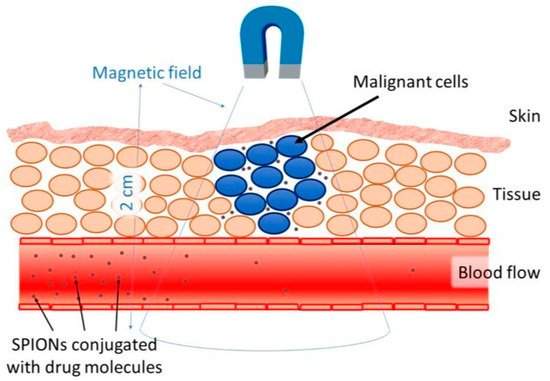
Targeted delivery methods are segregated into two categories, passive and active. Passive methods do not require external forces, whereas active methods require an external energy source to guide the nanoparticles to their site of action [52][91]. The effectiveness of the targeted delivery method is dependent on a variety of variables. For instance, the injection procedure, MNP concentration, hydrodynamic conditions, the qualities of the magnetic field, MNPs (includes drug-particle binding), and the target area’s location and depth [24]. One of the major issues with the application of MNPs is the depth in which the magnetic field is able to penetrate. The magnetic field can penetrate the body up to 2 cm from the skin without difficulty (Figure 5), however penetrating the body beyond 2 cm is difficult, since the magnetic field reduces with distance [30]. Nevertheless, internal magnets can be situated in the tumour’s proximity through minimally invasive surgery to evade the restrictions of external magnetic fields. Multiple studies have shown simulated interactions between MNPs and magnetic implants that enabled drug delivery. With respect to all biomedical applications, there are issues relating to the extrapolation of data from animal models to humans. There are multiple physiological measures that complicate this, such as variations in weight and cardiac output. However, a great deal of interest in this area persists, considering the accessibility nanoparticles provide to specific tumour sites, in comparison to conventional surgery [25].

In recent years, fluorescent magnetic nanoparticles (fluro-MNPs) were presented to be great vectors for drug delivery. Their size and concentration in tumours provide precise mapping of lesions and very high resolution, signifying their importance in biomedical applications. This combination of magnetic and fluorescent qualities in nanoparticles is significant for multi-functional contrast agents in medical bio-imaging [53][92].
The production of fluro-MNPs in an aqueous solution was performed with the addition of fluorescein iso-thiocyanate (FITC) during fabrication. Fluorescence spectrophotometry and confocal microscopy detected an intensity of fluorescence in fluro-MNPs, in comparison to MNPs developed without FITC. To be appropriate for drug delivery, the fluro-MNPs were functionalized by five layers of PEG/CMC. The future use of fluro-MNPs for medical fluorescence bio-imaging and anti-cancer drug delivery is promising, given that fluro-MNPs have demonstrated that their surface is appropriate for functionalization [53][92].
4. Application of Magnetic Nanoparticles in Magnetic Hyperthermia
Hyperthermia is the gradual increase in temperature to 40–43 °C. It leads to the destruction of cancer cells and improves the outcomes of chemotherapy and radiation. The drawback of this approach is its inability to heat cancer cells locally. However, this issue can be bypassed by the intravenous administration of MNPs targeted towards specific sites, and the use of an external magnetic field to generate heat at a local level. This targeted technique could enhance the safety and efficiency of hyperthermia, since it does not cause damage to healthy neighbouring tissues [30].
Magnetic hyperthermia is a non-invasive approach to cancer therapy [22]. This approach offers an alternative for certain cancers that may be difficult to surgically remove and for those that reside proximally to vital organs. Since magnetic hyperthermia resolves the issue of nonselective ionizing radiation associated with conventional radiotherapy, treatment options may broaden beyond certain tumours [54][93]. Magnetite and maghemite are the most utilized nanoparticle materials for magnetic hyperthermia [30]. Magnetite is one of the most significant magnetic materials, due to its high saturation magnetization and its ability to easily functionalize biomolecules [55][94]. Magnetic hyperthermia is dependent on a rise in temperature in the tumour site, either to 41–47 °C for the induction of apoptosis, or to 50 °C for the induction of necrosis [22]. Temperatures up to 42 °C may be referred to as mild hyperthermia and higher temperatures may be referred to as extreme hyperthermia. The heat from higher temperatures may result in alterations to the permeability of the cell membrane, immune system stimulation, the denaturation of proteins, cytoskeletal damage, and impairment of specific DNA repair processes [56][95]. By utilizing an environment similar to the tumour microenvironment, it was shown that magnetic hyperthermia requires a target temperature about 6 °C lower than exogenous hyperthermia in order to achieve equivalent cell death effects. It also presents with cytotoxic effects of greater significance. Cancer cells are deemed to be more susceptible to heat in comparison to healthy cells, as a result of their increased rate of metabolism. On a tissular level, a tumour’s ability to disperse heat is reduced due to its disordered vascular system. Increased temperatures also enhance cell sensitivity to alternative treatments such as chemotherapy and radiation [57][96]. Moreover, the increase in cancer cell sensitivity can be attributed to the improved anti-tumour immune response caused by hyperthermia due to its ability to enhance the presentation of tumour antigens, trafficking of leukocytes throughout the endothelium, and NK cell and dendritic cell activation [54][93]. The degree of sensitivity is dependent on the time taken between heating and chemotherapy, drug nature and concentration, tumour type, and tumour temperature [58][97]. Hyperthermia treatment induces certain biological effects within cancer cells, such as enhanced lysosomal permeability, which in turn culminates in an increase in oxidative stress due to the production of reactive oxygen species. The reduction of tumour cell viability through amplified cathepsin D activity within the cytoplasm is another consequence of increased lysosomal permeability. Furthermore, the rotation of SPIONs induced by dynamic magnetic fields may disrupt lipid membrane stability, thereby influencing lysosomal permeability and resulting in the activation of apoptosis [54][93].
Brownian and Néel relaxation explain the rise in temperature found in particles exposed to an external magnetic field, during the process of magnetic hyperthermia [59][98]. Neel relaxation converts energy from an external magnetic field into heat generation and Brownian relaxation results in the rotation of MNPs leading to cell damage [60][99]. The alternating magnetic field permeates through tissue, allowing tumours to be treated in various positions within the body. The effectiveness of heating is dependent on the particle’s response to an external magnetic field, the frequency and amplitude of the magnetic field, and particle size. The particle’s resistance against the magnetic field, generates heat within the particles. The particle’s transfer of energy, magnetic to thermal, can be measured as a specific absorption rate (SAR) [22]. The injected dose administered to the patient reduces as the SAR increases [57][96].
The value of SAR is dependent on the frequency and amplitude of the magnetic field, the shape and size of the particles, as well as their magnetic qualities. A study stated that graphene oxide adjustable magnetic nanorods applied in mice models were efficacious for hyperthermia. The 350 nm nanorods had the greatest SAR value of 1045 W g−1 at 0.2 mg mL−1 of iron concentration, amongst the three various nanorods that were utilized (250, 350, and 460 nm). The 350 nm nanorods displayed an adequate decrease in tumour volume in the mice model and demonstrated favourable biocompatibility in the MTT assay [22]. Intrinsic loss parameter (ILP) is an additional concept to consider. A number of researchers began reporting ILP instead of SAR because it eliminates the influence of field and frequency in the calculation. ILP is only deemed constant in conditions of low field strength and low frequency measurements. Consequently, utilizing ILP as a comparative tool between studies is not advised. In favour of successfully comparing SAR performances of samples, the field, frequency, media, and measured concentration must all be kept constant [61][100].
In recent times, in-vivo experiments were implemented to research the effects of magnetic hyperthermia on the size of tumours. A group of researchers utilized carboxydextran coated superparamagnetic iron oxide nanoparticles for magnetic hyperthermia. BALB/c nu/nu athymic mice injected with non-small cell lung cancer cell line A549 cells were exposed to an alternating magnetic field for 20 min. The tumour size was reported to be significantly reduced. Correspondingly, researchers injected murine pancreatic carcinoma cell line Pan02 cells into mice to synthesize pancreatic cancer tissue. Iron oxide nanoparticles loaded onto monocyte/macrophage-like cells were injected into the mice following tumour growth. After three days, the MNPs found in cancerous tissue generated heat upon exposure to an alternating magnetic field. After a period of time, a substantial decline in tumour size was recorded [62][101]. Additionally, Sadhuka et al. have recently demonstrated that hyperthermia aids in the eradication of cancer stem cell populations, which are a significant cause of metastasis and the recurrence of cancer. This could be due to the production of reactive oxygen species [63][102].
Despite its initial clinical application, hyperthermia has presented various drawbacks that should be addressed for its implementation to become widespread. For hyperthermia to compete with conventional cancer therapy, synergetic effects must be demonstrated via its application with radiotherapy and chemotherapy, however its limited effectiveness hinders this process. Uninhibited heat dispersion results in reduced effectiveness, which is mainly due to the absence of powerful devices capable of controlling and surveilling local temperatures. This may be worsened by the distance between the heat source and the target tumour cells, the circulatory system producing thermal dissipation, and the inhomogeneity of the tissues. Since hyperthermia is more complicated than standard therapies, efforts have been made for the development of new specific equipment for hyperthermia treatment. In addition, hyperthermia may cause local side-effects as the heat source is not completely adjacent to the cancer cells being treated. Consequently, undesirable toxicity may occur within healthy cells or tissues due to the increase in temperature. Therefore, it is essential that thermal biological studies are undertaken to gain a further understanding. The thermotolerance and the effect of hyperthermia on cells on a molecular level, including the sensitives of various cancer types to temperature, should also be assessed [54][93].
These issues may be tackled through the utilization of a contactless stimulus that localizes heat induction and via the enhancement of the real-time control of temperature. Magnetic resonance thermometry (MRT) is a successful non-invasive MRI-based technique that includes the measurement of temperature distributions in 3D, potentially replacing the current utilization of invasive thermal probes. Forty years ago, the combined use of MRI with thermometry was suggested. MRT techniques have since enhanced their accuracy for their application in vivo and pre-clinical developmental stages. Novel non-invasive targeted sources of heat have been investigated for hyperthermia to further increase its effectiveness and to evade undesired side-effects. Although a single equipment enabling the simultaneous performance of both hyperthermia and in-vivo location would be advantageous, the varying magnetic field requirements between MRI and hyperthermia prohibit guidance in real-time [54][93].
