Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jason Zhu and Version 1 by Zheng Jin.
Herein, a novel chitosan derivative nanoparticle was proposed to function as a delivery carrier. First of all, an improvement was made to the way N-2-hydroxypropyl trimcthyl ammonium chloride chitosan (N-2-HACC) was synthesized.
- Nanoparticles
- chitosan
- Immune
- N-2-HACC
1. Introduction
Porcine epidemic diarrhea virus (PED) is a pig infectious disease caused by porcine epidemic diarrhea virus (PEDV), causing in particular a large number of death of piglets [1]. PEDV is transmitted by infecting porcine epithelial cells through the fecal–oral route [2]. Vaccine is an important way to prevent this disease [3]. To contain viral transmission, an ideal vaccine is supposed to induce mucosal immune responses. Existing vaccines are ineffective in the control and prevention of PEDV, which makes it necessary to develop high-efficiency vaccines. However, aluminum or mineral oil adjuvants are commonly used as vaccine adjuvants, which have certain toxic and side effects [4]. Polymer nanoparticles, especially nanoparticles of chitosan (CS) and its derivatives, as vaccine adjuvants are the focus of current research [5]. In addition, the nanoparticles of chitosan (CS) and its derivative can induce mucosal immune response, as confirmed in those relevant reports. [6,7][6][7].
However, the widespread application of CS has been restricted by its low solubility above pH 6.5 [8]. CS is not soluble in water, dilute alkali solution, or common organic solvents, but only in low-concentration inorganic acid acidic solutions such as dilute acetic acid, dilute hydrochloric acid, and some particular organic solvents. This is attributed to the stereoregularity of its molecular structure and the hydrogen bonding between molecules, which hinders its practical application [9,10,11][9][10][11]. In order to break down the H-bond network of CS and improve the solubility of CS, the introduction of functional groups into CS free amine groups or primary hydroxyl groups for chemical modification has now become the focus of attention for research [12]. The commonly used techniques of modification include carboxymethylation [13], quaternization [12], sulfation [14], and thiolation [15]. These artificially introduced functional groups endow CS with new functional properties, which makes CS and CS derivatives attract much attention for use in various medical and industrial settings [16]. Thus, in order to improve the water solubility of CS, while widening the scope of its application, CS was modified by quaternization and synthesized quaternized chitosan in previous studies, which is N-2-HACC, thus improving the water solubility of the modified CS derivative significantly [17]. Preparation of CS and CS derivative nanoparticles can be performed by cross-linking polymer chains together with covalent bonds, or by exploiting physical interactions between the polymer chains, such as hydrogen bonds, electrostatic forces, or hydrophobic associations. The attractive forces that can be exploited in the preparation of the particles may lead to both contraction and aggregation of the particles over time. Estimation of the compactness of the nanoparticles, along with their size, can help to discriminate between intra- and interparticle associations [18]. CS is readily soluble in an acidic environment due to protonation of the amine groups. The resultant positive charge makes it possible to prepare nanoparticles by ionotropic gelation with multivalent anions, such as tripolyphosphate (TPP) [19]. The same reaction can be achieved if there is a certain amount of retention of amine groups in CS derivatives, but TPP can be irritating to eyes and skin, which limits its use. Researchers propose to replace TPP with β-glycerophosphate sodium (β-GC). In addition, it is understood that there is no relevant report on the application of N-2-HACC and β-GC to the preparation of nanoparticles in the field of vaccines. However, the introduction of hydroxypropyl trimethyl ammonium chloride group will reduce the number of NH2 functional groups on N-2-HACC, thus affecting the effect of crosslinking into nanoparticles. The way in which to ensure good water solubility at a lower degree of substitution is an urgent problem to be solved in the preparation of N-2-HACC.
2. Materials
Chitosan (CS) with the molecular weight of 713 kDa and deacetylation degree of 85% was purchased from Sinopharm Chemical Reagent Co. Ltd, while acetic acid (AR) and β-glycerophosphoric (β-GC) acid disodium salt was provided by Tianli Chemical Reagent Co., Ltd. Sodium hydroxide (NaOH) and isopropanol (IPA) were purchased from Yongda Chemical Reagent Co., Ltd.; 2,3-epoxypropyl trimethyl ammonium chloride (EPTAC) was sourced from Dibo Biotechnology Co., Ltd.; and anhydrous ethanol was obtained from Fuyu Fine Chemical Co., Ltd.
3. Synthesis of the N-2-HACC
First of all, 6 g of CS was dissolved in acetic acid solution (66.6 mL acetic acid + 173.4 mL deionized water). Secondly, the pH value of the above liquid was adjusted to 9 with 15 mol/L NaOH solution for 0.5 h of soaking. Thirdly, the precipitate was washed to neutral with deionized water, and then dried in a vacuum freeze dryer (SJIA-10N-80C, Ningbo Shuangjia Instrument Co., Ltd., China). Fourthly, the freeze-dried product was dispersed in 50 mL of isopropanol solution, 80 °C water bath, with EPTAC isopropanol solution (9 g/50 mL) added dropwise within 30 min, wherein the reaction was 9 h and 150 mL ice-cold anhydrous ethanol was added; then, the mixture was soaked for 0.5 h, suction filtered, and vacuum freeze-dried to constant weight. The yield (Y) was gravimetrically determined using the following equations:
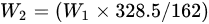
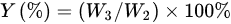
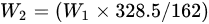
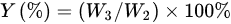
where W1 (g) represents the added quality of CS, W2 (g) indicates the theoretical generation quality of N-2-HACC, W3 (g) denotes the actual generation quality of N-2-HACC, 162 indicates the molecular weight of the CS monomer, and 328.5 refers to the molecular weight of the N-2-HACC monomer.
4. Nanoparticle Synthesis and Immune Effect Evaluation
First of all, 2 mL of sorbitan monooleate (span80), 10 mL of petroleum ether, and 20 mL liquid paraffin as the oil phase were added into a beaker. Then, 3 mL of N-2-HACC as the water phase was added into the beaker dropwise and stirred at 900 r/min for 15 min. Next, 1 mL β-GP was added and stirred for 4 min at the same speed, so as to obtain a pre-emulsion, which was then poured into a rapid emulsification device. The pre-emulsion penetrated the membrane (with a pore size of 0.45 μm) 3 times under a nitrogen pressure of 0.15 MPa, before curing in a 37 °C water bath for 3 h. Finally, the solution was centrifuged at 15,285× g for 10 min at 4 °C, with the supernatant removed. This was repeated three times for lyophilization. Diluted to varying concentrations at 0.5%, 1%, 5%, and 10% (w/v) and mixed with the same amount of PEDV after inactivation, N-2-HACC NPs were stirred at a high speed of 8916× g for 30 min to obtain the N-2-HACC/PEDV inactivated vaccine with different doses of N-2-HACC NPs. The vaccine components of different N-2-HACC NPs supplemented doses are shown in Table 1. Care of laboratory animals and experimentation on animals were in accordance with animal ethics guidelines and approved protocols. All the animal studies were approved by the Experimental Animal Ethics Committee of Heilongjiang University (ethic approval number: 20190304001). A total of 25 healthy guinea pigs with negative PEDV serum antibodies were randomly divided into 5 groups. The injection doses are detailed in Table 1 With each group of guinea pigs raised in the same environment, the second booster of immunization was administered in the same way 15 days after the first-time vaccination. After immunization, blood was collected on a weekly basis to detect the antibody titer of ELISA in the serum, and the trend of antibody changes in ELISA was observed to determine the optimal dosage of N-2-HACC NPs. The N-2-HACC solution of the optimal adjuvant dose as determined above was taken for the thorough mixing with the inactivated PEDV, and then stirred at 8916× g for 30 min. Finally, the N-2-HACC/PEDV inactivated vaccine was produced.Table 1. The vaccine composition with different adjuvant doses.
| Group | N-2-HACC NPs (%, | w | / | v | ) | PEDV (mL) | N-2-HACC NPs (g/mL) | Adjuvant Dose (mL) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 1 | 0.01 | 1 | ||||
| 2 | 1 | 1 | 0.02 | 1 | ||||
| 3 | 5 | 1 | 0.1 | 1 | ||||
| 4 | 10 | 1 | 0.2 | 1 |
5. N-2-HACC/PEDV Immune Effect
Figure 1 shows the immune effects of N-2-HACC/PEDV inactivated vaccines at varying adjuvant dosages. Since the prepared N-2-HACC/PEDV (107.0 TCID50/mL) was a pink liquid, it was easily separated from the bottle wall by gentle shaking. From the above results, the ELISA antibody titer of each adjuvant group was higher than that of the control group. Except for the 0.5% (w/v) adjuvant group, the ELISA antibody titer of each adjuvant group was significantly different to the control group. After 10 weeks of immunization, each adjuvant group reached their maximum, with the antibody titer of the 10% (w/v) adjuvant group found to be the highest, followed by the 5% (w/v) adjuvant group. In contrast, the antibody titer of the two groups remained at a high level. Compared with previous reports, the N-2-HACC/PEDV inactivated vaccines prepared by us had less PEDV (1%) mixed in proportion, and the immune effect and immune time were better, indicating that the N-2-HACC/PEDV inactivated vaccines is an effective PEDV candidate vaccine [37][20].
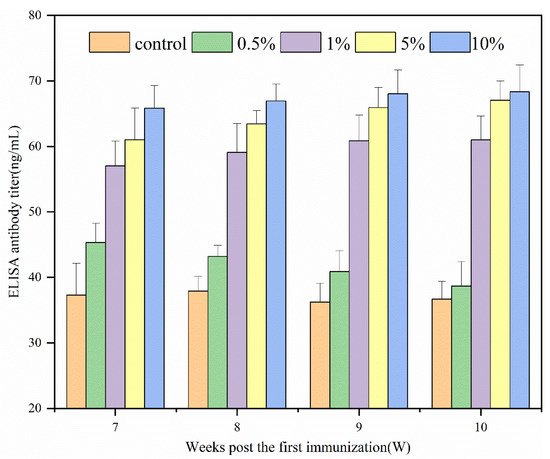


Figure 1. ELISA antibody dynamics regularity of serum in guinea pigs.
6. Preparation of N-2-HACC NPs
As can be seen from Figure 2, N-2-HACC NPs prepared under optimized conditions had uniform particle size, good dispersion, and regular morphology.

Figure 2. TEM morphology of N-2-HACC NPs.
Average diameter of N-2-HACC NPs was 215.9 ± 44.0 nm, and zeta potential was + 32.31 ± 0.65 mV. As reported, the size of the nanoparticles affects the absorption of the nanoparticles by immune cells. Nanoparticles similar in size to pathogens (5–300 nm for viruses) are easily absorbed by antigen-presenting cells, which enhance the immune response [35][21]. In addition, zeta potential is an important influencing factor in mucosal drug delivery, while positively charged polymers are conducive to adsorbing negatively charged mucins, enhancing mucosal adhesion, and extending the drug residence time [36][22]. Therefore, the N-2-HACC NPs was expected to deliver drugs to the mucosa and achieve local administration.
References
- Jung, K.; Saif, L.J.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045.
- Jung, K.; Saif, L.J. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015, 204, 134–143.
- Song, D.; Moon, H.; Kang, B. Porcine epidemic diarrhea: A review of current epidemiology and available vaccines. Clin. Exp. Vaccine Res. 2015, 4, 166–176.
- Shi, S.; Zhu, H.; Xia, X.; Liang, Z.; Ma, X.; Sun, B. Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 2019, 37, 3167–3178.
- Jin, Z.; Gao, S.; Cui, X.; Sun, D.; Zhao, K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int. J. Pharm. 2019, 572, 118731.
- Moine, L.; Canali, M.M.; Porporatto, C.; Correa, S.G. Reviewing the biological activity of chitosan in the mucosa: Focus on intestinal immunity. Int. J. Biol. Macromol. 2021, 189, 324–334.
- Mukhtar, M.; Fényes, E.; Bartos, C.; Zeeshan, M.; Ambrus, R. Chitosan biopolymer, its derivatives and potential applications in nano-therapeutics: A comprehensive review. Eur. Polym. J. 2021, 160, 110767.
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283.
- Liang, Y.; Deng, L.; Chen, C.; Zhang, J.; Zhou, R.; Li, X.; Hu, R.; Dong, A. Preparation and properties of thermoreversible hydrogels based on methoxy poly(ethylene glycol)-grafted chitosan nanoparticles for drug delivery systems. Carbohydr. Polym. 2011, 83, 1828–1833.
- Shelma, R.; Sharma, C.P. Submicroparticles composed of amphiphilic chitosan derivative for oral insulin and curcumin release applications. Colloids Surf. B Biointerfaces 2011, 88, 722–728.
- Yan, D.; Hu, S.; Zhou, Z.; Zeenat, S.; Cheng, F.; Li, Y.; Feng, C.; Cheng, X.; Chen, X. Different chemical groups modification on the surface of chitosan nonwoven dressing and the hemostatic properties. Int. J. Biol. Macromol. 2018, 107, 463–469.
- Andreica, B.-I.; Cheng, X.; Marin, L. Quaternary ammonium salts of chitosan. A critical overview on the synthesis and properties generated by quaternization. Eur. Polym. J. 2020, 139, 110016.
- Chakka, V.P.; Zhou, T. Carboxymethylation of polysaccharides: Synthesis and bioactivities. Int. J. Biol. Macromol. 2020, 165, 2425–2431.
- Jayakumar, R.; Nwe, N.; Tokura, S.; Tamura, H. Sulfated chitin and chitosan as novel biomaterials. Int. J. Biol. Macromol. 2007, 40, 175–181.
- Kazemi, M.S.; Mohammadi, Z.; Amini, M.; Yousefi, M.; Tarighi, P.; Eftekhari, S.; Rafiee Tehrani, M. Thiolated chitosan-lauric acid as a new chitosan derivative: Synthesis, characterization and cytotoxicity. Int. J. Biol. Macromol. 2019, 136, 823–830.
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702.
- Wang, C.-H.; Liu, W.-S.; Sun, J.-F.; Hou, G.-G.; Chen, Q.; Cong, W.; Zhao, F. Non-toxic O-quaternized chitosan materials with better water solubility and antimicrobial function. Int. J. Biol. Macromol. 2016, 84, 418–427.
- Dashtimoghadam, E.; Mirzadeh, H.; Taromi, F.A.; Nyström, B. Microfluidic self-assembly of polymeric nanoparticles with tunable compactness for controlled drug delivery. Polymer 2013, 54, 4972–4979.
- Koukaras, E.N.; Papadimitriou, S.A.; Bikiaris, D.N.; Froudakis, G.E. Insight on the Formation of Chitosan Nanoparticles through Ionotropic Gelation with Tripolyphosphate. Mol. Pharm. 2012, 9, 2856–2862.
- Li, B.; Du, L.; Yu, Z.; Sun, B.; Xu, X.; Fan, B.; Guo, R.; Yuan, W.; He, K. Poly (d,l-lactide-co-glycolide) nanoparticle-entrapped vaccine induces a protective immune response against porcine epidemic diarrhea virus infection in piglets. Vaccine 2017, 35, 7010–7017.
- Prasanth Koppolu, B.; Smith, S.G.; Ravindranathan, S.; Jayanthi, S.; Suresh Kumar, T.K.; Zaharoff, D.A. Controlling chitosan-based encapsulation for protein and vaccine delivery. Biomaterials 2014, 35, 4382–4389.
- Feng, C.; Li, J.; Kong, M.; Liu, Y.; Cheng, X.J.; Li, Y.; Park, H.J.; Chen, X.G. Surface charge effect on mucoadhesion of chitosan based nanogels for local anti-colorectal cancer drug delivery. Colloids Surf. B Biointerfaces 2015, 128, 439–447.
More
