Among cardiovascular diseases, acute myocardial infarction (AMI) shows significant differences in occurrence rate, prognosis and efficacy of treatment between male and female patients. Genomics and epigenomics approaches together with epidrugs design and drug repositioning could fill the sex-gap.
- gender medicine
- sex disparities
- genetics/molecular biomarkers
- molecular medicine
- OMICS
- genomics
1. Introduction
In the recent past, there has been a growing attention to sex-based differences in biology, genetics, biomedical sciences and general medicine, ranging from the cellular level to whole organs and organisms. As expected, this process quickly led to the generation of new insights into diagnostic, prognostic and therapeutic issues, from basic research to the clinical level [17][1]. The overall message is indeed the one published by Nature in 2010, which summarizes old and new problems in the title “Putting gender on the agenda” [18][2]. Starting from the fact that animals have a sex [19][3], well known differences in gene expression have to exist in male versus female mice [20][4]. Based on the evidence that companies and scientists may have arbitrarily performed their preclinical tests on male models, the Editors of Nature concluded that “Medicine as it is currently applied to women is less evidence-based than that being applied to men” [18][2]. The increasing attention towards sex and gender, along with the interest that emerges from this kind of aware research, are now beginning to bridge the gap [21][5]. Thanks to the increased knowledge of the molecular, genomics and epigenomics bases of complex diseases, and thanks to the personalized pharmacogenetic/genomics approach to drug design/prescription, several diseases are now faced in a tailored fashion [22,23][6][7]. However, while the inclusion of sex is a process already underway, with evident results from both preclinical and clinical trials, the impact of gender in medical/biomedical fields is still at an early stage, with difficulties and delays due to its intrinsic complexity. Ongoing efforts aim to include and understand the role of gender in pharmacology [24,25][8][9]. To date, gender-related pharmacodynamic and pharmacokinetic differences have been reported with crucial implications on drugs effects [26,27,28,29,30][10][11][12][13][14]. Overall, gender-specific pre-clinical models will increase the definition of gender-oriented therapeutic protocols, in turn accelerating the development of gender-specific drugs and the generation of gender-oriented and evidence-based guidelines [11,31][15][16].
2. Sex Disparity
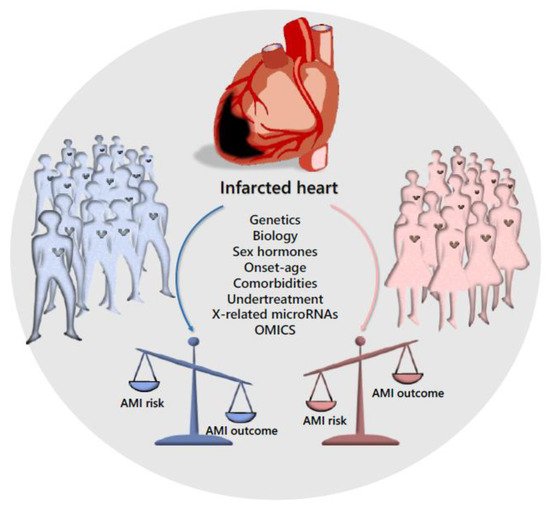
References
- Legato, M.J.; Johnson, P.A.; Manson, J.E. Consideration of Sex Differences in Medicine to Improve Health Care and Patient Outcomes. JAMA 2016, 316, 1865–1866.
- Putting gender on the agenda. Nature 2010, 465, 665.
- Blaustein, J.D. Animals have a sex, and so should titles and methods sections of articles in Endocrinology. Endocrinology 2012, 153, 2539–2540.
- Yang, X.; Schadt, E.E.; Wang, S.; Wang, H.; Arnold, A.P.; Ingram-Drake, L.; Drake, T.A.; Lusis, A.J. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006, 16, 995–1004.
- Oertelt-Prigione, S.; Dalibert, L.; Verdonk, P.; Stutz, E.Z.; Klinge, I. Implementation Strategies for Gender-Sensitive Public Health Practice: A European Workshop. J. Womens Health 2017, 26, 1255–1261.
- Kaminsky, Z.; Wang, S.C.; Petronis, A. Complex disease, gender and epigenetics. Ann. Med. 2006, 38, 530–544.
- Sioud, M.; Melien, O. Treatment options and individualized medicine. Methods Mol. Biol. 2007, 361, 327–340.
- Franconi, F.; Raparelli, V.; Regitz-Zagrosek, V. Sex and gender landscape in pharmacology. Pharmacol. Res. 2017, 123, 93–94.
- Rodriquez, M.; Aquino, R.P.; D’Ursi, A.M. Is it time to integrate sex and gender into drug design and development? Future Med. Chem. 2015, 7, 557–559.
- Franconi, F.; Campesi, I. Sex and gender influences on pharmacological response: An overview. Expert Rev. Clin. Pharmacol. 2014, 7, 469–485.
- Basili, S.; Raparelli, V.; Proietti, M.; Tanzilli, G.; Franconi, F. Impact of sex and gender on the efficacy of antiplatelet therapy: The female perspective. J. Atheroscler. Thromb. 2015, 22, 109–125.
- Di Giosia, P.; Passacquale, G.; Petrarca, M.; Giorgini, P.; Marra, A.M.; Ferro, A. Gender differences in cardiovascular prophylaxis: Focus on antiplatelet treatment. Pharmacol. Res. 2017, 119, 36–47.
- Pucci, G.; Alcidi, R.; Tap, L.; Battista, F.; Mattace-Raso, F.; Schillaci, G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol. Res. 2017, 120, 34–42.
- Campesi, I.; Franconi, F.; Seghieri, G.; Meloni, M. Sex-gender-related therapeutic approaches for cardiovascular complications associated with diabetes. Pharmacol. Res. 2017, 119, 195–207.
- Franconi, F.; Rosano, G.; Campesi, I. Need for gender-specific pre-analytical testing: The dark side of the moon in laboratory testing. Int. J. Cardiol. 2015, 179, 514–535.
- Franconi, F.; Campesi, I.; Colombo, D.; Antonini, P. Sex-Gender Variable: Methodological Recommendations for Increasing Scientific Value of Clinical Studies. Cells 2019, 8, 476.
- Available online: http://www.worldheartfailure.org/WHFS (accessed on 24 October 2019).
- Available online: http://www.medicographia.com/2012/02/the-heart-failure-epidemic (accessed on 24 October 2019).
- Mehta, L.S.; Beckie, T.M.; DeVon, H.A.; Grines, C.L.; Krumholz, H.M.; Johnson, M.N.; Lindley, K.J.; Vaccarino, V.; Wang, T.Y.; Watson, K.E.; et al. Acute Myocardial Infarction in Women: A Scientific Statement From the American Heart Association. Circulation 2016, 133, 916–947.
- Davis, E.; Gorog, D.A.; Rihal, C.; Prasad, A.; Srinivasan, M. “Mind the gap” acute coronary syndrome in women: A contemporary review of current clinical evidence. Int. J. Cardiol. 2017, 227, 840–849.
- Oertelt-Prigione, S. Gender and cardiovascular disease in the workplace—It’s not just about pay gaps. Int. J. Cardiol. 2018, 262, 108–109.
- Myocardial Infarction Genetics Consortium; Kathiresan, S.; Voight, B.F.; Purcell, S.; Musunuru, K.; Ardissino, D.; Mannucci, P.M.; Anand, S.; Engert, J.C.; Samani, N.J.; et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat. Genet. 2009, 41, 334–341.
- Nakatochi, M.; Ichihara, S.; Yamamoto, K.; Naruse, K.; Yokota, S.; Asano, H.; Matsubara, T.; Yokota, M. Epigenome-wide association of myocardial infarction with DNA methylation sites at loci related to cardiovascular disease. Clin. Epigenet. 2017, 9, 54.
- Webb, T.R.; Erdmann, J.; Stirrups, K.E.; Stitziel, N.O.; Masca, N.G.; Jansen, H.; Kanoni, S.; Nelson, C.P.; Ferrario, P.G.; Konig, I.R.; et al. Systematic Evaluation of Pleiotropy Identifies 6 Further Loci Associated With Coronary Artery Disease. J. Am. Coll. Cardiol. 2017, 69, 823–836.
- Chen, R.; Snyder, M. Promise of personalized omics to precision medicine. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 73–82.
- Baetta, R.; Pontremoli, M.; Fernandez, A.M.; Spickett, C.M.; Banfi, C. Reprint of: Proteomics in cardiovascular diseases: Unveiling sex and gender differences in the era of precision medicine. J. Proteom. 2018, 178, 57–72.
- Chaudhary, R.; Sukhi, A.; Chaudhary, R.; Jindal, M.; Vyas, A.; Rout, A.; Bliden, K.; Tantry, U.; Gurbel, P. Gender differences in thrombogenicity among patients with angina and non-obstructive coronary artery disease. J. Thromb. Thrombolysis 2019.
- Patel, R.; Tragante, V.; Schmidt, A.F.; McCubrey, R.O.; Holmes, M.V.; Howe, L.J.; Direk, K.; Akerblom, A.; Leander, K.; Virani, S.S.; et al. Subsequent Event Risk in Individuals with Established Coronary Heart Disease: Design and Rationale of the GENIUS-CHD Consortium. Circ. Genom. Precis. Med. 2019.
- Gemmati, D.; Serino, M.L.; Trivellato, C.; Fiorini, S.; Scapoli, G.L. C677T substitution in the methylenetetrahydrofolate reductase gene as a risk factor for venous thrombosis and arterial disease in selected patients. Haematologica 1999, 84, 824–828.
- Gemmati, D.; Serino, M.L.; Ongaro, A.; Tognazzo, S.; Moratelli, S.; Resca, R.; Moretti, M.; Scapoli, G.L. A common mutation in the gene for coagulation factor XIII-A (VAL34Leu): A risk factor for primary intracerebral hemorrhage is protective against atherothrombotic diseases. Am. J. Hematol. 2001, 67, 183–188.
- Campo, G.; Valgimigli, M.; Ferraresi, P.; Malagutti, P.; Baroni, M.; Arcozzi, C.; Gemmati, D.; Percoco, G.; Parrinello, G.; Ferrari, R.; et al. Tissue factor and coagulation factor VII levels during acute myocardial infarction: Association with genotype and adverse events. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2800–2806.
- Gemmati, D.; Federici, F.; Campo, G.; Tognazzo, S.; Serino, M.L.; De Mattei, M.; Valgimigli, M.; Malagutti, P.; Guardigli, G.; Ferraresi, P.; et al. Factor XIIIA-V34L and factor XIIIB-H95R gene variants: Effects on survival in myocardial infarction patients. Mol. Med. 2007, 13, 112–120.
- Gemmati, D.; Zeri, G.; Orioli, E.; Mari, R.; Moratelli, S.; Vigliano, M.; Marchesini, J.; Grossi, M.E.; Pecoraro, A.; Cuneo, A.; et al. Factor XIII-A dynamics in acute myocardial infarction: A novel prognostic biomarker? Thromb. Haemost. 2015, 114, 123–132.
- Gemmati, D.; Vigliano, M.; Burini, F.; Mari, R.; El Mohsein, H.H.; Parmeggiani, F.; Serino, M.L. Coagulation Factor XIIIA (F13A1): Novel Perspectives in Treatment and Pharmacogenetics. Curr. Pharm. Des. 2016, 22, 1449–1459.
- Ansani, L.; Marchesini, J.; Pestelli, G.; Luisi, G.A.; Scillitani, G.; Longo, G.; Milani, D.; Serino, M.L.; Tisato, V.; Gemmati, D. F13A1 Gene Variant (V34L) and Residual Circulating FXIIIA Levels Predict Short- and Long-Term Mortality in Acute Myocardial Infarction after Coronary Angioplasty. Int. J. Mol. Sci. 2018, 19, 2766.
- De Luca, L.; Marini, M.; Gonzini, L.; Boccanelli, A.; Casella, G.; Chiarella, F.; De Servi, S.; Di Chiara, A.; Di Pasquale, G.; Olivari, Z.; et al. Contemporary Trends and Age-Specific Sex Differences in Management and Outcome for Patients With ST-Segment Elevation Myocardial Infarction. J. Am. Heart Assoc. 2016, 5.
- Dreyer, R.P.; Ranasinghe, I.; Wang, Y.; Dharmarajan, K.; Murugiah, K.; Nuti, S.V.; Hsieh, A.F.; Spertus, J.A.; Krumholz, H.M. Sex Differences in the Rate, Timing, and Principal Diagnoses of 30-Day Readmissions in Younger Patients with Acute Myocardial Infarction. Circulation 2015, 132, 158–166.
- Dreyer, R.P.; Dharmarajan, K.; Kennedy, K.F.; Jones, P.G.; Vaccarino, V.; Murugiah, K.; Nuti, S.V.; Smolderen, K.G.; Buchanan, D.M.; Spertus, J.A.; et al. Sex Differences in 1-Year All-Cause Rehospitalization in Patients After Acute Myocardial Infarction: A Prospective Observational Study. Circulation 2017, 135, 521–531.
- Writing Group, M.; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e48.
- Wells, G.L. Cardiovascular Risk Factors: Does Sex Matter? Curr. Vasc. Pharmacol. 2016, 14, 452–457.
- Dunlay, S.M.; Roger, V.L. Gender differences in the pathophysiology, clinical presentation, and outcomes of ischemic heart failure. Curr. Heart Fail. Rep. 2012, 9, 267–276.
- Seeland, U.; Regitz-Zagrosek, V. Sex and gender differences in cardiovascular drug therapy. Handb. Exp. Pharmacol. 2012, 211–236.
- Ranasinghe, I.; Wang, Y.; Dharmarajan, K.; Hsieh, A.F.; Bernheim, S.M.; Krumholz, H.M. Readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia among young and middle-aged adults: A retrospective observational cohort study. PLoS Med. 2014, 11, e1001737.
- Bauters, C.; Dubois, E.; Porouchani, S.; Saloux, E.; Fertin, M.; de Groote, P.; Lamblin, N.; Pinet, F. Long-term prognostic impact of left ventricular remodeling after a first myocardial infarction in modern clinical practice. PLoS ONE 2017, 12, e0188884.
- Subramanya, V.; Zhao, D.; Ouyang, P.; Lima, J.A.; Vaidya, D.; Ndumele, C.E.; Bluemke, D.A.; Shah, S.J.; Guallar, E.; Nwabuo, C.C.; et al. Sex hormone levels and change in left ventricular structure among men and post-menopausal women: The Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas 2018, 108, 37–44.
- Ter Horst, E.N.; Hakimzadeh, N.; van der Laan, A.M.; Krijnen, P.A.; Niessen, H.W.; Piek, J.J. Modulators of Macrophage Polarization Influence Healing of the Infarcted Myocardium. Int. J. Mol. Sci. 2015, 16, 29583–29591.
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac Extracellular Vesicles in Normal and Infarcted Heart. Int. J. Mol. Sci. 2016, 17, 63.
- Musial-Wysocka, A.; Kot, M.; Sulkowski, M.; Majka, M. Regenerative Potential of the Product “CardioCell” Derived from the Wharton’s Jelly Mesenchymal Stem Cells for Treating Hindlimb Ischemia. Int. J. Mol. Sci. 2019, 20, 4632.
- Sun, T.; Dong, Y.H.; Du, W.; Shi, C.Y.; Wang, K.; Tariq, M.A.; Wang, J.X.; Li, P.F. The Role of MicroRNAs in Myocardial Infarction: From Molecular Mechanism to Clinical Application. Int. J. Mol. Sci. 2017, 18, 745.
- Singh, A.V.; Subhashree, L.; Milani, P.; Gemmati, D.; Zamboni, P. Interplay of iron metallobiology, metalloproteinases, and FXIII, and role of their gene variants in venous leg ulcer. Int. J. Low Extrem. Wounds 2010, 9, 166–179.
- Zamboni, P.; Gemmati, D. Clinical implications of gene polymorphisms in venous leg ulcer: A model in tissue injury and reparative process. Thromb. Haemost. 2007, 98, 131–137.
- Zamboni, P.; De Mattei, M.; Ongaro, A.; Fogato, L.; Carandina, S.; De Palma, M.; Tognazzo, S.; Scapoli, G.L.; Serino, M.L.; Caruso, A.; et al. Factor XIII contrasts the effects of metalloproteinases in human dermal fibroblast cultured cells. Vasc. Endovasc. Surg. 2004, 38, 431–438.
- Tognazzo, S.; Gemmati, D.; Palazzo, A.; Catozzi, L.; Carandina, S.; Legnaro, A.; Tacconi, G.; Scapoli, G.L.; Zamboni, P. Prognostic role of factor XIII gene variants in nonhealing venous leg ulcers. J. Vasc. Surg. 2006, 44, 815–819.
- Gemmati, D.; Tognazzo, S.; Catozzi, L.; Federici, F.; De Palma, M.; Gianesini, S.; Scapoli, G.L.; De Mattei, M.; Liboni, A.; Zamboni, P. Influence of gene polymorphisms in ulcer healing process after superficial venous surgery. J. Vasc. Surg. 2006, 44, 554–562.
- Gemmati, D.; Tognazzo, S.; Serino, M.L.; Fogato, L.; Carandina, S.; De Palma, M.; Izzo, M.; De Mattei, M.; Ongaro, A.; Scapoli, G.L.; et al. Factor XIII V34L polymorphism modulates the risk of chronic venous leg ulcer progression and extension. Wound Repair Regen. 2004, 12, 512–517.
- Gemmati, D.; Occhionorelli, S.; Tisato, V.; Vigliano, M.; Longo, G.; Gonelli, A.; Sibilla, M.G.; Serino, M.L.; Zamboni, P. Inherited genetic predispositions in F13A1 and F13B genes predict abdominal adhesion formation: Identification of gender prognostic indicators. Sci. Rep. 2018, 8, 16916.
- Greiten, L.E.; Holditch, S.J.; Arunachalam, S.P.; Miller, V.M. Should there be sex-specific criteria for the diagnosis and treatment of heart failure? J. Cardiovasc. Transl. Res. 2014, 7, 139–155.
