Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Silvia Giovannini and Version 2 by Vicky Zhou.
Sarcopenia is a progressive, systemic musculoskeletal disorder associated with an increased risk of adverse events such as falls and fractures, mobility disorders, cardiac and respiratory disease, cognitive impairment, institutionalization, and death. Physical disability and impaired ability to perform activities of daily living contribute to reducing both patient quality of life and functional independence, adding to the necessity of long-term care services for the patient. Considering this evidence, it would seem clear that early diagnosis of sarcopenia and care optimization would also reduce the economic impact on the health care system and individual social-economic burdens.
- ultrasound
- Sarcopenia
1. Introduction
Numerous studies and research groups have been working over the past ten years to provide a precise definition of sarcopenia. Five major research groups around the world have developed different definitions and diagnostic criteria: the European Working Group on Sarcopenia in Older People (EWGSOP), the Asia Working Group for Sarcopenia (AWGS), the International Working Group on Sarcopenia (IWGS), the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project, and the Society for Sarcopenia Cachexia and Wasting Disorders (SCWD). As a result, in 2016, sarcopenia was added to the International Classification of Diseases with its ICD-10-MC diagnostic code [1][2][5,6]. In 2018, the European Working Group on Sarcopenia in Older People (EWGSOP2) published the revised “European Consensus on Sarcopenia” introducing a new working definition, based on low muscle strength as the primary parameter of sarcopenia, considering it a more reliable measure of muscle function and a better predictor of adverse outcomes. Behind sarcopenia, there are mechanisms that in some cases overlap with some cell aging processes. In particular, there are alterations in protein synthesis, proteolysis, neuromuscular integrity, and muscle fat content [3][4][5][7,8,9]. It would be considered that sarcopenia is common in significative fragile patients, often affected by multimorbidity, hospitalization, and consumption of several drugs [6][7][10,11].
Screening and diagnosis of sarcopenia are important to prevent adverse health outcomes. CT and MRI are the gold standards for noninvasively assessing muscle quantity/mass and identifying adipose tissue [8][9][12,13]. However, these examinations are expensive, expose patients to radiation, are not always available, and in some situations are difficult to perform and thus must be performed in the hospital. DXA is more easily accessible but less accurate [10][1].
In the last few years, new evidence has shown that ultrasound provides detailed imaging about morphology and size and of the surrounding structures [11][14]. Several studies reported ultrasound as an appropriate tool to evaluate muscle characteristics and morphology in sarcopenia [12][13][14][15,16,17].
Moreover, ultrasonography is low cost, simple to use, community or hospital-based, is easily repeatable, and is widely used.
The age-related biochemical, anatomical, and physiological changes in muscle tissue have been known for some time. These alterations occur at the molecular and cellular levels and are reflected in macroscopic changes evident on ultrasound. Alterations in muscle architecture reflect underlying sarcopenia, which causes muscle dysfunction. These changes have been recognized as good imaging biomarkers based on ultrasonography [15][18].
The quality and quantity of muscle tissue can be easily assessed with ultrasound by using some specific parameters.
In particular, the evaluation of pennate muscles is a potentially valuable tool. In clinical practice, it can highlight even minimal changes over a short period [16][19].
Ultrasonography is an accurate method even for use in the elderly, is easy to access, and can also be performed bedside inside the hospital or in the community [17][20].
Given the emerging role of ultrasonography, standardization of the method has recently been proposed. However, a global evidence-based consensus is still absent [18][21].
Ultrasonography, in the setting of screening and diagnosis of sarcopenia, has the potential to become an imaging-based tool comparable to CT, MRI to quantify body composition on the tissue level, and DXA on the chemical level.
At present, there are no pharmacological treatments available that are effective in the management of sarcopenia [19][22], whether they are hormone therapy or drugs. The frontier of sarcopenia treatment, especially in the elderly patient, is a personalized patient-centric approach. It is now known that there is interindividual variability at the cellular level and molecular expression [20][21][22][23,24,25]. This variability underlies alterations in different pathways and whose identity is the basis of the future sarcopenia management. Currently, only an adequate exercise regimen associated with correct nutritional intake (protein) has demonstrated efficacy in the management of sarcopenia. Of utmost importance is the prescription of resistance-based exercise, a diet rich in protein, and with an adequate caloric intake and vitamin supplements [23][26].
2. Skeletal Muscle Ultrasound in Sarcopenia
To diagnose sarcopenia in the elderly, evaluating skeletal muscle mass loss is one of the main elements. Although in recent years the importance of associating functional parameters that were to evaluate the loss of muscle performance in the elderly has been emphasized [10][1], the diagnosis of sarcopenia cannot ignore the precise measurement of skeletal muscle mass loss, both in quantitative and qualitative terms [9][24][13,43]. Therefore, the simultaneous evaluation of both these parameters is recommended in the muscle mass measurement phase. On the one hand, diagnostic methods, such as DXA and BIA, are limited to providing only quantitative information about the skeletal muscle structure; on the other hand, CT and MRI, while offering an overview, are burdened by the limitations in the field of everyday clinical practice. In this sense, skeletal muscle ultrasound offers a valid alternative, as it is closely correlated with MRI [25][26][44,45], with CT [27][46] and DXA [28][29][47,48] in terms of measuring skeletal muscle mass and at the same time offering both quantitative and qualitative information. Although further studies on the standardization of ultrasound measurement methods are needed, the first step in this direction has been provided by the SARCUS study (SARCopenia through UltraSound) [18][21].
Ultrasonography (US) can effectively assess the quantity and quality of muscle tissue and has a certain degree of correlation with DXA in muscle study [29][30][48,49]. Some US parameters measure muscle quantity, such as muscle volume (MV), cross-sectional area (CSA), and muscle thickness (MT). Other parameters describe the muscle qualitatively, for example, echo intensity (EI), pennation angle (PA), fascicle length (FL), and physiologic cross-sectional area (PCSA). There are some experimental approaches, such as the measurement of vascularity.
These parameters can be studied at various “regional sites”; however, the one most frequently used site is the anterior compartment of the thigh. In addition, sarcopenia is not uncommonly site-specific. Muscle loss in the lower limbs is more pronounced in the mentioned district [31][32][50,51].
The technique involves supine positioning of the subject with legs extended. A linear probe (5–12 MHz) is used, with a fixed gain, and transverse and longitudinal images are taken at the quadriceps femoris. CSA, MT, and EI are assessed on transverse images. PA and FL are measured longitudinally. MT can be performed on any muscle; firstly, the evaluation involves the quadriceps femoris; CSA is evaluated mainly in the rectus femoris. MT and CSA have a good degree of correlation on muscle quantity with the other main methods for studying muscle (MRI, CT, and DXA) [33][26][34][41,45,52]. However, CSA or MT are subject to variability in the muscle explored, to “regional” sarcopenia. Not all anatomical regions undergo muscle loss at the same rate, so there may be some degree of discordance.
Among qualitative measures, muscle echo intensity (EI) is a parameter that has been recently studied. Granted that the amount of muscle does not directly correlate with function [35][32][29,51], muscle echogenicity provides helpful information about the presence of inflammation, fibrosis, and adipose tissue infiltration [36][53]. Increased intramuscular adipose tissue, also referred to as “myostatosis”, is a finding present in both aging-related sarcopenia and cancer cachexia [37][54]. These tissue rearrangements can cause an increase in muscle echogenicity, similar to findings in myopathies [38][55].
In addition, there appears to be a correlation between echo intensity and muscle strength, gate speed, and sit-to-stand test [39][40][41][56,57,58].
Alterations in architecture are crucial in the genesis of force and are parameters related to muscle function.
The pennation angle is formed by the insertion of muscle fibers at the level of the deep and superficial aponeurosis in most locomotor muscles, such as the medial gastrocnemius (Figure 12). The pennation angle and fascicle length are generally evaluated at the level of the gastrocnemius medialis. These two techniques allow for information on the structure and architecture of the muscle and therefore allow for a correlation with the genesis of muscle strength [42][30].
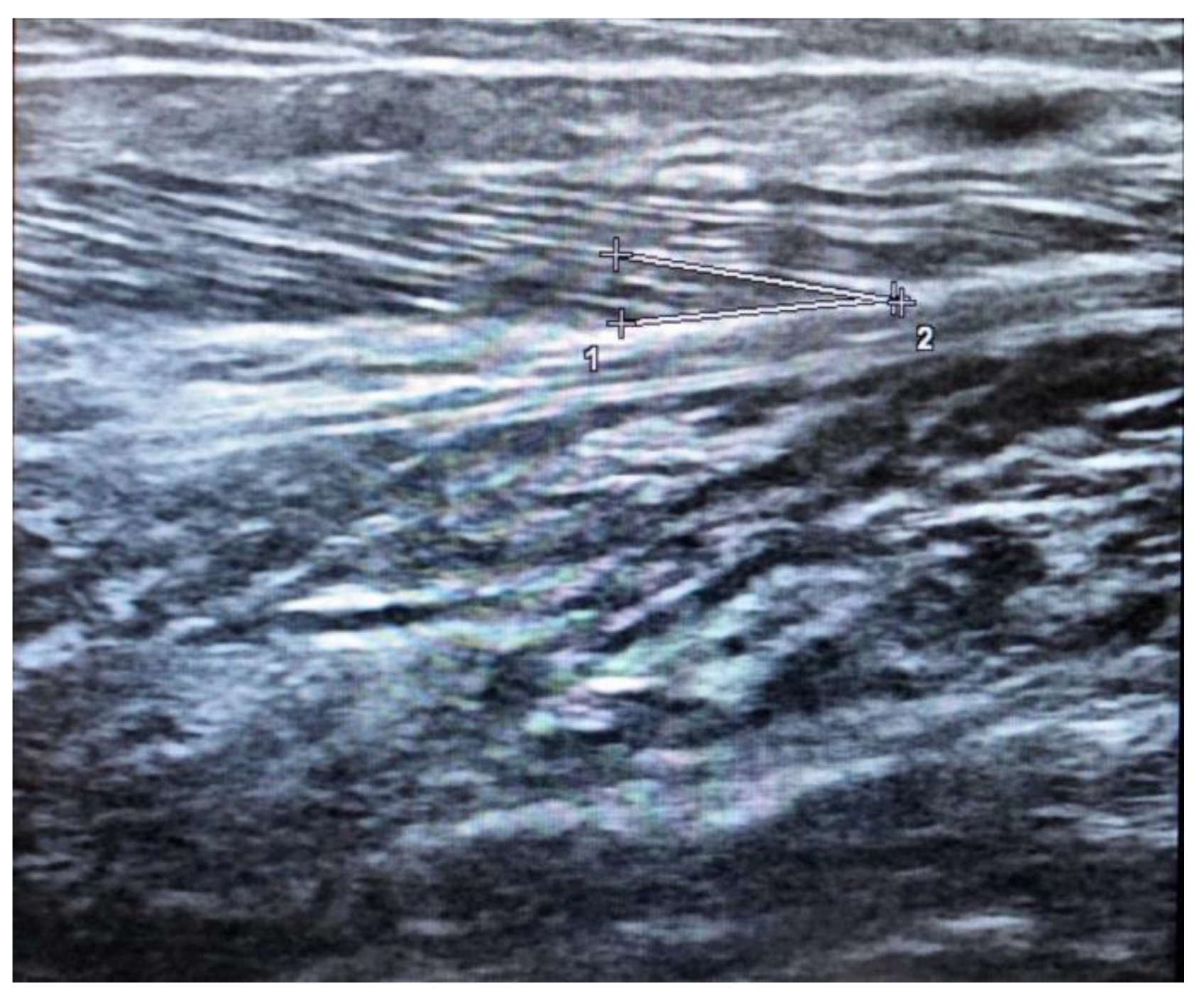

Figure 12. Ultrasound estimation of Pennation angle in the medial gastrocnemius given bu deep aponeurosis (1-2) and fascicle region (+).
However, these parameters are highly dependent on the technique used to scan. The fascicle length is the distance between the intersection composed of the superficial aponeurosis and the fascicle and the intersection composed of the deep aponeurosis and the fascicle.
These parameters present issues related to their use in long muscles. In such sections, the muscle fibers are longer than the actual field of view of classical US probes. In order to obtain a more accurate estimation, a reliable approach is to use extended field-of-view or to calculate FL from quantitative and qualitative measurements (MT and PA) [43][44][59,60].
Recently, standardization has been proposed, but a global consensus on the technique is currently missing [18][21]. The technique is also dependent on several US parameters (e.g., frequency, focus, gain) and muscle thickness [42][30]. However, it is possible, by quantitative evaluation, to eliminate this limitation [10][1].
Another important assessment is the physiologic cross-sectional area (PCSA); it is obtained primarily on quadriceps femoris scans and is derived from CSA, PA, and FL. It can help to assess muscle structure and its relationship to function. This parameter also allows for a more accurate estimate of the number of muscle fibers in each section.
The fascicle length and the pennation angle and their modifications are the main structural parameters that can be measured by ultrasound in skeletal muscles [45][61]. Narici and colleagues have shown that aging is associated with significant changes in the ultrasound structure of the medial gastrocnemius, with a reduced length of the muscle fascicle and pennation angle [46][62]. A decrease in physiological cross-section is related to muscle function more than the anatomical cross-sectional area measured by CT or MRI [46][62]. A study showed that the pennation angle and the cross-section area of the lateral head of the gastrocnemius muscle in a group of healthy older adults were smaller than the control in young subjects [47][63].
Additional qualitative analysis is the study of muscle vascularization; it is generally assessed at the level of the quadriceps femoris muscle. This study allows for the identification of areas at potential risk of sarcopenia, which can be visualized as alterations in the vasculature. Microvascular damage and nitric oxide deficiency are thought to underlie the pathogenesis of sarcopenia [48][49][64,65]. However, this technique requires a high level of expertise, data processing software, and special materials.
Finally, muscle stiffness is the relation between the possible degree of deformation and compression of the muscle [45][61]. These factors are determined by connective tissue, such as extracellular matrix collagen, which provides passive tension, and muscle contraction, which produces active tension. A recent study has suggested that changes in muscle stiffness, as measured by elastography, could be linked to muscle weakness [46][62].
EWGSOP2 included in the definition of sarcopenia the detection of a poor quantity and quality of muscle tissue. Thus, the combined evaluation of quantitative (CSA and MT) and qualitative (EI, FL, and PA) parameters could be important to identify sarcopenia; US evaluates in a simple and easily repeatable way any changes over time, both in terms of the evolution of sarcopenia and in terms of evaluation of the response to a given intervention. The use of additional biomarkers complementary to the US could increase the accuracy of the method itself [50][66].
3. Conclusions
The application of ultrasound in the assessment of sarcopenia is helpful, especially for detecting muscle failure in older patients not being influenced by the presence of acute and chronic disease and fluid balance. Despite small intra and inter-individual variability, this technique could characterize sarcopenia through the study of qualitative and quantitative parameters measured in different muscles groups, particularly in lower limb muscles. Ultrasound provides a quantitative and qualitative study of muscle, has several modalities, and can study the muscle tissue of multiple anatomical regions. For the evaluation of sarcopenia, state of the art ultrasonography had the potential to be comparable to the other main methodologies. The recent attempts to standardize the technique, the low cost, the simplicity of execution, the absence of ionizing radiation, the reproducibility, and repeatability make it a possible method to be used in the near future for the diagnostic assessment and treatment response in sarcopenia. Further studies will be needed to confirm and standardize the use of the methodology in a clinical setting.
In conclusion, having limited options for sarcopenia management, the best strategy is prevention and early diagnosis. With this perspective, determining the future role of ultrasound in this field will be crucial.
