Osteoporosis, a metabolic skeletal disorder that results from the imbalance between bone formation and bone resorption, generally occurs in postmenopausal women and older people. The process of bone remodeling is participated by mainly osteoclasts and osteoblasts, together with other cells including osteocytes, bone lining cells, monocytes, chondrocytes, hematopoietic stem cells, and mesenchymal stem cells (MSCs).
- osteoporosis
- mesenchymal stem cells
- extracellular vesicles
- EV cargo
- engineering EV
- osteoporosis medications
1. MSC-EV with Carried Molecules for Diseases Treatment
Extracellular vesicles (EVs) are lipid bound, nano- to micrometer scaled vesicles secreted by almost all cell types. They can be divided into three subgroups by size, biogenesis, release pathways, encapsulated content and function: exosomes (30–200 nm), microvesicles (MVs, 45–1000 nm), and apoptotic bodies (ABs, 1–4 μm) [1][2][28,29].
Based on the guideline of MISEV (minimal information for studies of extracellular vesicles) 2018, “extracellular vesicle (EVs)” is an expert consensus term to describe the vesicles that have the characteristics as follows: (1) The vesicles cannot replicate. (2) The vesicles are naturally released from the cell. (3) The vesicles are encapsulated by lipid bilayers. EVs should be characterized by at least three positive markers (one transmembrane/lipid-bound protein is included) with one negative marker. The markers like tetraspanins families (e.g., CD9, CD63, CD81, and CD82), MVB membrane transport (Alix and TSG101), and heat-shock proteins (Hsp70 and Hsp90) are commonly used as EVs’ markers [3][4][30,31]. In particular, MSCs-EVs can reflect the markers from their parental cells by expression of CD29, CD44, CD73, and CD90. This cell-type fingerprint not only provides the targets for characterization, but also indicates that MSC-EVs have a similarly potential to MSCs in the treatment of various disease [5][6][32,33]. Among these EVs, exosomes have drawn great attention due to the therapeutic potential and medical application of exosomes from certain cell types, such as MSCs or other stem cells. Exosomes are encapsulated by a lipid bilayer membrane with several types of molecules within the exosomal membrane, including integrins, adhesion molecules, lipid, and certain receptors. Inside the exosomes, various types of molecules are encapsulated, including DNA, messenger RNA, microRNA, non-coding RNA, enzymes, cytokines, as well as many other proteins [7][14]. All these cargos are the materials involved in the cell–cell communication through exosomes trafficking and uptake by recipient cells. Moreover, different types of cells produce exosomes with different content of cargo, which have different effects. There has been strong evidence that exosomes are the main carrier in charge of transporting most of the secreted factors from cells [8][15]. Currently, exosomes represent an important mode of intercellular communication by serving as vehicles for transfer between cells of membrane and cytosolic proteins, lipids, and RNA [9][16].
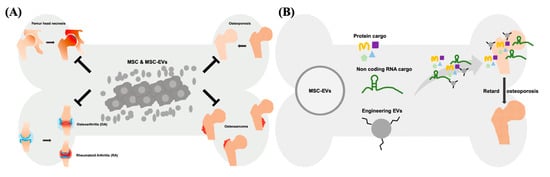
The therapeutic effect of EVs can be exerted via horizontal transfer of molecules, such as proteins, lipids and several types of nucleic acids [10][11][12][34,35,36]. Recently, the therapeutic potential of noncoding RNAs (ncRNAs) has drawn a lot of attention. These ncRNAs include microRNAs (miRNAs), Piwi-interacting RNA (piRNA), long non-coding RNAs (lncRNAs), and others types of secretomes derived from cells. Among these ncRNAs, certain miRNAs and lncRNAs have shown therapeutic potential in numerous diseases [13][14][15][37,38,39]. miRNAs are regulatory small RNAs with 21–23 nt that are involved in posttranscriptional downregulation of protein [16][40]. They target and inhibit the translation of specific mRNAs and eventually influence the gene expression profile as well as cellular behavior. As the result, EVs’ miRNAs are generally considered the crucial therapeutic cargos in the MSC-EV-based therapy [17][18][19][41,42,43]. The majority of the mammalian genome is transcribed into non-protein-coding mRNAs, including lncRNAs [20][44]. In the cytoplasm, lncRNAs served as the competing endogenous RNA (ceRNA) to control the binding of miRNA-mRNA, and mediate the translational regulation of mRNA, as well as function as scaffolds of RNA-protein complexes [21][22][45,46]. Nuclear lncRNAs mainly influence the organization of chromatin by interacting with related proteins, or prevent the gene loci targeted from specific chromatin factors [23][47]. As the potential therapeutic targets, several studies demonstrated that lncRNAs play an important role in the treatment of cancer, rare diseases, and infectious diseases [24][48]. For example, lncRNA H19 carried by MSC-EVs can be transferred to fibroblasts, upregulates the PTEN signaling pathway, and stimulate wound healing in diabetic foot ulcers [25][49]. The protein cargos of MSC-EVs can be associated with several biological processes of various diseases, particularly with tissue repair and regeneration [26][50]. EVs derived from WJ-MSCs showed the immunomodulatory capability, mainly through TGF-β and adenosine carried in the EVs which suppress the activation of CD4-expressing T-cells and used for the treatment of canine diseases [27][51]. Overexpression of hypoxia inducible factor (HIF)-1α in the human dental pulp MSCs promotes the release of EVs, concomitantly carrying overexpressed HIF-1α. These EVs promote angiogenesis by interacting with Notch signaling-rated protein (Jagged1) [28][52]. Both MSCs and MSC-EVs attenuate ureteral fibrosis by inhibition of TGF-β1/Smad signaling pathway, whereas the therapeutic effect of MSCs might attribute to EVs, the paracrine factors secreted by transplanted MSCs [29][53]. Of note, MSCs and MSC-EVs act as medications for several bone diseases, such as femur head necrosis, OA, RA, osteosarcoma, and osteoporosis via transferring therapeutic cargo ( Figure 1 ). Collectively, MSC-EVs have shown the curative potential not less than that of parental MSCs in the treatment of numerus disease models, indicating that MSC-EVs is a worth developing medication in the future.
2. Applications of Modified EVs for Therapy
In general, the terms “modification” and “engineering” are used to describe the biotechnologies involved in the alteration of the materials for cells, particles, and vesicles [30][31][32][54,55,56]. The common materials include chemical linkers, ligands, proteins, lipids, and nucleic acids. Further, the alteration induced by external pressure such as hypoxia environment, mechanical force, sonochemical reactions, and voltage operations are recognized as the approaches of modification or engineering [33][34][35][57,58,59]. At the genetic level, the term “genetic modification or engineering” can comprehensively cover the process of intentional alteration of genes to produce a beneficial characteristic to a targeted organism [36][60]. Nevertheless, for the broad spectrum of biomaterials including EVs, nanoparticles (NPs), liposomes, cells, and tissues, there is no uniform description of “modification” and “engineering”. In this review, both “modification” and “engineering” are used to describe the introduction of external materials to EVs or alteration induced by application of pressure. There are a plethora of methods to modify EVs, and loading of exogenous proteins or nucleic acids into EVs are common methods used to explore the feasibility of modified EVs for disease treatment. Physical approaches, including saponin permeabilization, extrusion, sonication, and freeze/thaw cycles, were reported to load hydrophilic molecule (catalase) into EVs [37][61]. To increase the quantity of EVs, cells cultured in the hypoxic environment secreted EVs with 1.5–2 folds more than that of cells cultured normoxia [38][62]. Other methods of EV engineering such as surface modification, phospholipid-domain binding, click chemistry, and hybrid EVs with liposomes are introduced to enhance the ability of binding, targeting, and stability of EVs and potentiate the therapeutic effect [39][40][41][42][43][63,64,65,66,67]. However, the major challenge of EV engineering is keeping the biological functions of EVs when loading materials or modifying. Several studies showed that loading of exogenous nucleic acids into EV by electroporation might induce the aggregation of EVs and the loaded materials, lower the loading efficiency, and reduce the uptake of EVs by target cells [44][45][68,69]. Sonication is an effective method for active loading of hydrophilic agents or nucleic acids into EVs. However, cargo of nucleic acids is easily degraded due to their structural nature and the adverse effect may be resulted by sonication [46][47][70,71]. The cargo can be introduced into EVs by incubating with transfection reagent. However, EV membrane might be altered by reagent, which might further affect the delivery of EVs [48][49][72,73]. Taken together, suitable modification and engineering of EVs strengthen the therapeutic potential through different aspects, such as enhanced targeting ability or loading of therapeutic agents. Nevertheless, engineering without affecting the bioactivity and function of EVs is always a critical concern.
3. Therapeutic Potential of Exogenous MSC-EVs for Osteoporosis
To date, the strategies of using MSCs or MSC-EVs in the treatment of metabolic bone diseases mainly focus on regulating the bone remodeling by promotion of osteoblasts and inhibition of osteoclasts. Numerous important molecules are involved in the bone remodeling and therefore can serve as the markers for the measurement of dynamic change. These include alkaline phosphatase (ALP), RUNX Family Transcription Factor 2 (RUNX2), collagen, type I, alpha 1 (COL1A1), collagen, type I, alpha 2 (COL1A2), Osteopontin (OPN), osteocalcin (OCN, BGLAP), osterix (Osx, SP7), cathepsin K (CTSK), tartrate-resistant acid phosphatase (TRAP), and calcitonin receptor (CALCR) [50][51][52][74,75,76]. In addition to these markers, there are also signaling pathways regulating the bone remodeling, such as WNT/β-catenin, transforming growth factor-betas (TGF-β), bone morphogenetic proteins (BMP), insulin-like growth factors (IGF), phosphoinositide 3-kinase (PI3K)/Akt, and RANKL/RANK/OPG signaling pathways [53][54][55][56][57][77,78,79,80,81]. With the examination of these protein markers or signaling pathways, MSC-EVs are demonstrated to be benefit in the induction of osteogenesis or suppression of osteoclastogenesis. For instance, treatment of EVs derived from human dental pulp stem cells (hDPSCs) promoted the osteogenesis of adipose-derived stem cells (hADSCs) by targeting to MAPK pathway [58][82]. EVs derived from osteogenic differentiated hADSCs showed enhanced ability to induce osteogenesis of hADSCs. This beneficial loop was verified by upregulated expression of ALP and RUNX2 [59][83]. In terms of osteoclastogenesis, EVs derived from gingival tissue-derived MSCs (GMSCs) was reported to target to Wnt5a-mediated RANKL pathway, and inhibit the activity and the number of osteoclasts. This therapeutic effect was enhanced after GMSCs are pretreated with tumor necrosis factor alpha (TNF-α) [60][84]. Despite the promising results from these preclinical studies, so far only few clinical trials of MSC-EVs in the treatment of bone diseases are registered and conducted, as compared with that of MSCs ( Table 1 ). This suggests that the application of MSC-EVs for bone disorders is still at the initial stage, and more evidence regarding the therapeutic effect, targeted signaling pathways, and other mechanisms are needed.
| No. | NCT04501354 | NCT04499105 | NCT04414592 | NCT04759105 | NCT05066334 | NCT04297813 | NCT03692221 | NCT04735185 | No. | NCT04849429 | NCT04998058 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase | Mesenchymal stem cells (MSCs) | Phase 2 | Phase 2 | Not Applicable |
Phase 2 | Phase 2 | Phase 3 | Early Phase 1 | Not Applicable |
Phase | Extracellular vesicles (EVs) | Phase 1 | Phase 1 |
| Intervention/treatment | Mesenchymal Stem Cell | Mesenchymal Stem Cell + NaCl 0,9% 2 mL | Mesenchymal stem cells | Mesenchymal stem cells | Mesenchymal stem cells | Combination Product: Advanced medicinal Therapy (MSC combined with biomaterial) Procedure: Autologous bone graft |
Mesenchymal stem cells | Other: Autologous stem cells Drug: CorticosteroidDrug: Local anesthetic |
Intervention/treatment | Biological: Platelet rich plasma (PRP) with exosomes | Procedure: Maxillary sinus floor elevation grafting with synthetic bone substitute. | ||
| Intervention model | Single Group Assignment | Single Group Assignment | Single Group Assignment | Parallel Assignment | Parallel Assignment | Parallel Assignment | Parallel Assignment | Parallel Assignment | Intervention Model | Parallel Assignment | Parallel Assignment | ||
| Cell sources | Umbilical cord | Umbilical cord | Human umbilical cord | Bone marrow | Bone marrow | - | Bone marrow | Bone marrow | EV sources/term | Platelet-rich Plasma/Exosomes | Adipose tissue-derived mesenchymal stem cells/Conditioned medium | ||
| Condition or disease | Osteoporosis | Degenerative Disc DiseaseLow Back Pain Disc Degeneration |
Lumbar Disc Degeneration Lumbar Disc Herniation |
Intervertebral Disc Degeneration Chronic Low-back Pain |
Intervertebral Disc Degeneration Chronic Low-back Pain |
Alveolar Bone Atrophy | Disc Degeneration | Chronic Low Back Pain Degenerative Disc Disease |
Condition or disease | Chronic Low Back Pain Degenerative Disc Disease | Bone Loss, Osteoclastic Bone Loss, Alveolar Alveolar Bone Loss Alveolar Bone Atrophy Grafting Bone |
||
| Last update posted | 7 August 2020 | 6 August 2020 | 4 June 2020 | 18 February 2021 | 4 October 2021 | 12 March 2020 | 4 April 2019 | 10 May 2021 | Last Update Posted | 19 April 2021 | 10 August 2021 | ||
| Sponsor | Indonesia University | Indonesia University | Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine | Campus Bio-Medico University of Rome | Campus Bio-Medico University of Rome | University of Bergen | University Hospitals Cleveland Medical Center | Johns Hopkins University | Sponsor | Dr. Himanshu Bansal Foundation | Pontifical Catholic University of Rio Grande do Sul |
For the treatment of osteoporosis, several studies have indicated the therapeutic potential of miRNA in MSC-EVs. A report showed that in the MSC-EVs, 171 miRNAs were discovered which regulated at least 5481 genes and subsequently influenced numerous signaling pathways [61][85]. After OVX-rats were treated with EVs derived from human bone marrow MSCs (hBMSC-EVs), the study found the increased expression of miR-551b, miR-1263, miR-181b, miR-144, miR-21, and miR-186 in the bone tissues of OVX-rats. Among these, miR-186 was verified to inhibit the expression of MOB Kinase Activator 1A (Mob1) and YAP, which act as the mediators in Hippo signaling pathway [62][86]. hBMSC-EVs also showed the therapeutic effect with retardation of osteoporosis, by delivery of miR-29b to ovariectomized (OVX) mice. According to the report, MSC-EVs isolated from osteoporotic patients lacked the expression of miR-29b-3p, and therefore the investigators introduced miR-29b-3p into EVs for the treatment of OVX mice. They found that miR-29b-3p-encapsulated EVs targeted and suppressed the nuclear factor kappa B (NF-κB) signaling pathway by inhibiting KDM5A expression, and subsequently improved the osteoporosis in OVX mice [63][87]. Similar to EVs from bone marrow MSCs, EVs from Wharton’s jelly MSCs have strong potential in the treatment of osteoporosis via miRNA cargo. A study (preprint published on Research Square, DOI: 10.21203/rs.3.rs-37420/v1) proclaimed that WJMSC-EVs’ miR-328-3p and miR-2110 promoted osteogenesis of osteoporotic mice. The report also showed that the PPAR (peroxisome proliferator activated receptor) signaling pathway-associated miR-2110 and let-7c-5p were enriched in WJ-MSC-EVs and regulated osteoclastogenesis [64][88]. Except for the OVX mice/rats model, osteoporosis treatment by MSC-EVs was also effective in the aged male mice model. EVs secreted from human umbilical cord blood (UCB-EVs) ameliorated bone loss in 16-month-old mice, and it might attribute to the repression of Homeobox A2 by EVs’ miR-3960. Once Homeobox A2, which 1is known to suppress RUNX2 expression, is downregulated by mir-3960, the promotion of osteoblast differentiation will be activated [65][66][89,90]. Another group showed that treatment of mouse BMSCs derived EVs had limited benefit in the increase of bone index of healthy mice, including bone mineral density (BMD), trabecular bone volume (BV/TV), trabecular bone number (Tb.N), and trabecular separation (Tb. Sp) as examined by microCT. However, when loaded with miR-29a, these EVs significantly induced osteogenesis in healthy mice. Further in this study, miR-29a was verified to improve osteoporosis and angiogenesis by directly target to VASH1 [67][91]. CBS +/− mice (CBS, Cystathionine β-synthase) tend to behave metabolic bone loss with increased level of homocysteine (Hcy), which is similar to the physical condition of osteoporotic postmenopausal women [68][92]. When osteoporotic CBS-heterozygous mice were treated with mouse BMSC-EVs, the result revealed that the angiogenesis and osteogenesis of mice were improved via the regulation by lncRNA-H19 (lnc-H19) in BMSC-EVs. Mechanistically, lnc-H19 bound to and inhibited miR-106, which was known to downregulated angiopoietin 1 ( Angpt1 ) with the function of bone formation stimulation [69][93]. Although the achievement of EVs’ miRNAs and lncRNAs based therapy for osteoporosis are impressive, several researches revealed contradictory results and provide another perspective in treatment for diseases by EVs non-coding RNAs cargo. The investigators isolated the EVs from various tissues including plasma, seminal fluid, dendritic cells, mast cells, and ovarian cancer cells for miRNAs quantitative assay, however, most individual EVs did not carry enough amounts of miRNAs to exert their function (According to the result of that research: 0.00825 ± 0.02 miRNA molecules/EV). Needless to say, the individual EV unlikely transfer miRNAs to the cells in their vicinity followed by participating in the down-stream signaling pathway [70][94].
To assess the therapeutic potential of protein carried by MSC-MVs, protein expression profiling performed by proteomic analysis is a useful tool for researchers. The proteomic signatures of MSC-EVs were revealed by extracting EVs from human bone marrow samples at 2012, the study identified 730 EV-carried proteins, and detailed the function into several pathways including MAPK, TGF-β, PPAR, BMP, Wnt, and GF signaling pathways [71][96]. Interestingly, most of mentioned pathways are related to the pathology of osteoporosis, it elucidated that utilized MSC-EVs protein cargo to develop osteoporosis medications were worthy to pursuit. With increasing studies for proteomic analysis of MSC-EVs, more than 3000 unique proteins have been identified to date. Although the variation of protein cargo in individuals might lead to the difficulties in drug development, the identified cargo still shares similar functional category that provide investigators clear targets to design MSC-EV’ protein-based pharmaceuticals [72][73][97,98]. Therefore, a number of researches reported the therapeutic potential of using MSC-EV’ protein cargo in treatment of osteoporosis. OVX mice were used in evaluation of therapeutic effect of human umbilical cord mesenchymal stromal cells-derived extracellular vesicles (hucMSC-EVs) for osteoporosis. A total of 5570 EV-carried proteins were identified by LC-MS/MS analysis, among those cargo, CLEC11A had the highest E/C ratio (EVs/parental cells ratio), allowing it to be a potential target in promotion of osteogenesis. Based on the hypothesis, the group successfully demonstrated that hucMSC-EVs retarded the osteoporosis via transferring CLEC11A. CLEC11A transferred by MSC-EVs not only stimulated progenitor cells to differentiate into osteoblasts, but also participated in regulation of osteoclastogenesis [74][99]. Moreover, CLEC11A was verified to bind Integrin α11 (Itga11) to stimulate osteogenic activity; however, the investigators did not examine whether CLEC11A carried by exogenous MSC-EVs bind to Itga11 to activate down-stream signaling or not [75][100]. Moreover, the BMSC-EVs obtained from SD rats were intravenously injected into OVX rats via the caudal vein. Significant improvement of BMD, BV/TV, Tb. N, and Tb. Sp values were revealed after the treatment. The authors further overexpressed the glycoprotein non-melanoma clone B (GPNMB), which facilitated osteoblast differentiation, in the BMSCs and released the EVs with high GPNMB expression (GPNMB-EVs). The result showed that GPNMB-EVs significantly promoted the osteogenesis in vitro and ameliorate the osteoporosis in vivo in comparison with control group (OVX mice without treatment) and BMSC-EVs transfected with empty vector group (NC-EVs) [76][101]. Given that the BMSC-EVs could attenuate osteoporosis by targeting to Wnt/β-catenin signaling pathway, the authors extended their research in exploration of possible mechanisms of treatment by GPNMB-EVs, and found out that GSK-3β might be the crucial molecule in the treatment. Currently, due to the obstruction of applying MSC in clinical studies, the alternative source of MSC has been explored [77][102]. With the characteristics of a noninvasive, low-cost, simple procedure, and massive production, the human urine-derived stem cells (USCs) are gradually used in research of stem cells-based therapy [78][103]. As the result, the therapeutic potential of USCs derived EVs(USC-EVs) for osteoporosis was assessed. Through the transfer of collagen triple-helix repeat containing 1(CTHRC1) and osteoprotegerin (OPG), USC-EVs effectively promoted the osteogenesis and suppressed the osteoclastogenesis to avoid OVX-induced osteoporotic mice from bone loss [79][104]. Although knockdown of CTHRC1 or OPG did not totally inhibit the therapeutic effect of USC-EVs due the promiscuous signaling pathway in bone remodeling, the research provided a strong evidence of using EVs’ protein cargo as osteoporosis medications despite USCs are not canonical type of MSC. To date, most studies demonstrated the beneficial effect of MSC-EVs for osteoporosis; however, the contradictory research showed that BMSC-EVs isolated from maxillary bones enhanced osteoclastogenesis [80][105].
