Hyaluronic acid (HA) is a non-sulphated glycosaminoglycan formed by repetitive units of glucuronic acid and N-acetyl glucosamine. HA is widely present in the extracellular matrix in vertebrates and invertebrates to confer mechanical support, viscoelastic and hygroscopic properties, and anti-inflammatory effects to cells and tissue. HA is one of the fundamental components of cartilage and tendon tissue, contributing to their viscoelastic properties.
- hyaluronic acid
- receptor
- tendon
- tendinopathy
- inflammation
- viscoelastic
- hygroscopic
- effect
1. Introduction
Hyaluronic acid (HA) is a non-sulphated glycosaminoglycan formed by repetitive units of glucuronic acid and N-acetyl glucosamine. HA is widely present in the extracellular matrix in vertebrates and invertebrates to confer mechanical support, viscoelastic and hygroscopic properties, and anti-inflammatory effects to cells and tissue. HA is one of the fundamental components of cartilage and tendon tissue, contributing to their viscoelastic properties [1,2,3,4,5][1][2][3][4][5]. HA enhances the cellular activities of fibroblasts, including their adhesivity, extracellular matrix (ECM) synthesis, and proliferation, but, despite its influence on cells and tendon structure, its role on the biomechanical function is still not clarified [6,7][6][7]. The ECM of tendons is predominantly composed of type I collagen ( Figure 1 ) and proteoglycans, with a small amount of the other types of collagen (type II, III, V, VI, IX, XI) ( Table 1 ). The predominant cells type within the tendon are tenoblasts or tenocytes, accounting for 90–95% of cells present in tendon tissue between collagen fibres [8]. Tenoblasts and tenocytes show different metabolic features: while tenoblasts have high activity, tenocytes are metabolically less active. Both types of cells produce collagen, elastin, ECM, and proteins [9]. Other types of cells present in lesser quantities include chondrocytes at the bone attachment and insertion sites, synovial cells in the tendon sheath, and vascular cells, capillary endothelial cells, and arterioles’ smooth muscle cells. All cells that produce the ECM are involved in endogenous HA production as well. HA injections are widely used to treat osteoarthritis (OA), but their efficacy in the management of tendinopathies is still debated [10]. Tendinopathies are characterised by tendon structure disruption, ineffective neovascularisation, decreased collagen I, and enhanced collagen II production [10]. Recently, the anti-inflammatory and viscoelastic effects of HA on connective tissue have been suggested to warrant the use of HA for the treatment of tendinopathies [11]. Some studies proved and support its use to improve function and reduce pain in tendinopathies [12[12][13],13], avoiding the complications of corticosteroids [14].
| ECM Components | % |
|---|---|
| Collagen | 86% (type I: 98%) |
| Proteoglycan | 1–5% |
| Elastin | 2% |
| Decorin | <1% |
| Aggrecan | <1% |
| Other proteins | <1% |
2. Patellar Tendinopathy and HA
Patellar tendinopathy is common in sports in which athletes jump, as in volleyball, basketball, or triple jump [91][15]. Kumai et al. evaluated the effects of a single high molecular weight HA injection in patients with patellar tendinopathy, finding improvement in pain and visual analogue scale (VAS) values at short-term follow-up (one week) [13]. Muneta et al. treated 50 young athletes with two high molecular weight HA injections without ultrasound (US) guidance. This was effective, safe, and repeatable [92][16]. Fogli et al. showed that a cycle of one HA injection a week for three weeks was effective and safe in patellar tendinopathy, with a significant decrease in pain, mean VAS values, and improvement of US appearance [93][17]. Kaux et al. compared US-guided injections of platelet-rich plasma (PRP) versus two HA injections, reporting that both treatments could be effective. The PRP group showed significant improvement in quadriceps strength, while HA had a greater impact on the improvement of symptoms [94][18]. Recently, Frizziero et al. showed good results after three medium molecular weight US-guided HA injections. This sentudry reported pain relief and improvement of the Victorian Institute of Sport Assessment for the patellar tendon (VISA-P) values at 90 days follow-up, with a decrease in vascularisation and tendon thickness at US and Power Doppler analysis [10].
3. Achilles Tendinopathy and HA
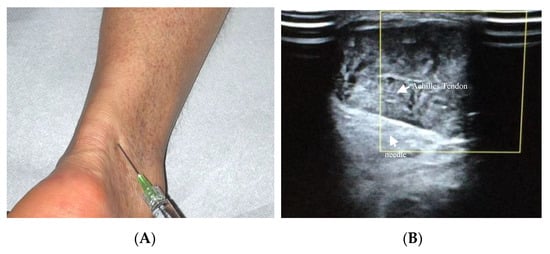
Achilles tendinopathy affects mainly athletes, especially runners, but also non-athletes [95][19]. The therapeutic use of HA in Achilles tendinopathy has been recently described ( Figure 32 A,B). Lynen et al. compared the effects of two HA peritendinous injections with shockwave therapy in patients with mid-portion Achilles tendinopathy. At 6 months, patients treated with HA injections reported better outcomes, with greater symptom improvement and restored function [14]. Similarly, Fogli et al. and Frizziero et al. showed that three US-guided medium molecular weight HA injections induce a clinically relevant increase in VISA-A values, pain relief, and US parameters improvement [10,93][10][17]. Recently, Gervasi et al. investigated the clinical, viscoelastometric, and biochemical effects of three US-guided medium molecular weight HA injections in runners with unilateral Achilles tendinopathy. Indeed, patients reported improvement in clinical assessment, decreased pain and stiffness of the tendon, and reduction in the viscoelastometric and functional asymmetry between the affected tendon and the healthy limb [96,97][20][21].
4. Epicondylitis and HA
Lateral epicondylitis is a common cause of chronic elbow pain and affects 1% to 3% of the general population per year [98][22]. The tendon structures most affected are the insertions of the extensor of the forearm, located on the lateral side of the elbow [98][22]. Petrella et al. compared outcomes in patients who received HA injections versus a control group, who received an injection of 1.2 mL of a saline placebo, finding significantly greater improvement in VAS pain at rest and after grip testing up to 1-year follow-up [99][23]. Khan et al. reported the efficacy of a single HA injection in the management of moderate epicondylitis (VAS pain score < 7), while it was not effective in severe lateral epicondylitis [100][24]. In a prospective randomised trial, Tosun et al. compared a combined HA-chondroitin sulphate injection versus a corticosteroid injection, showing an equal reduction in pain and improvement of function in the short term, while HA resulted in better outcomes at long-term follow-up [101][25].
References
- Balazs, E.A.; Laurent, T.C.; Jeanloz, R.W. Nomenclature of hyaluronic acid. Biochem. J. 1986, 235, 903.
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y. Signaling properties of hyaluronan receptors. J. Biol. Chem. 2002, 277, 4589–4592.
- Toole, B.P.; Wight, T.N.; Tammi, M.I. Hyaluronan-cell interactions in cancer and vascular disease. J. Biol. Chem. 2002, 277, 4593–4596.
- Hascall, V.C.; Majors, A.K.; De La Motte, C.A.; Evanko, S.P.; Wang, A.; Drazba, J.A.; Strong, S.A.; Wight, T.N. Intracellular hyaluronan: A new frontier for inflammation? Biochim. Biophys. Acta 2004, 1673, 3–12.
- Kogan, G.; Soltes, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25.
- Sawaguchi, N.; Majima, T.; Iwasaki, N.; Funakoshi, T.; Shimode, K.; Onodera, T.; Minami, A. Extracellular matrix modulates expression of cell-surface proteoglycan genes in fibroblasts. Connect Tissue Res. 2006, 47, 141–148.
- Sikes, K.J.; Renner, K.; Li, J.; Grande-Allen, K.J.; Connell, J.P.; Cali, V.; Midura, R.J.; Sandy, J.D.; Plaas, A.; Wang, V.M. Knockout of hyaluronan synthase 1, but not 3, impairs formation of the retrocalcaneal bursa. J. Orthop. Res. 2018, 36, 2622–2632.
- Hess, G.P.; Cappiello, W.L.; Poole, R.M.; Hunter, S.C. Prevention and Treatment of Overuse Tendon Injuries. Sports Med. 1989, 8, 371–384.
- Bolon, B. Mini-Review: Toxic Tendinopathy. Toxicol. Pathol. 2017, 45, 834–837.
- Frizziero, A.; Oliva, F.; Vittadini, F.; Vetrano, M.; Bernetti, A.; Giordan, N.; Vulpiani, M.; Santilli, V.; Masiero, S.; Maffulli, N.; et al. Efficacy of ultrasound-guided hyaluronic acid injections in achilles and patellar tendinopathies:a prospective multicentric clinical trial. Muscles Ligaments Tendon J. 2019, 09, 305.
- Kaux, J.F.; Samson, A.; Crielaard, J.M. Hyaluronic acid and tendon lesions. Muscles Ligaments Tendons J. 2015, 5, 264–269.
- Merolla, G.; Bianchi, P.; Porcellini, G. Ultrasound-guided subacromial injections of sodium hyaluronate for the management of rotator cuff tendinopathy: A prospective comparative study with rehabilitation therapy. Musculoskelet. Surg. 2013, 97 (Suppl. S1), 49–56.
- Kumai, T.; Muneta, T.; Tsuchiya, A.; Shiraishi, M.; Ishizaki, Y.; Sugimoto, K.; Samoto, N.; Isomoto, S.; Tanaka, Y.; Takakura, Y. The short-term effect after a single injection of high-molecular-weight hyaluronic acid in patients with enthesopathies (lateral epicondylitis, patellar tendinopathy, insertional Achilles tendinopathy, and plantar fasciitis): A preliminary study. J. Orthop. Sci. 2014, 19, 603–611.
- Lynen, N.; De Vroey, T.; Spiegel, I.; Van Ongeval, F.; Hendrickx, N.J.; Stassijns, G. Comparison of Peritendinous Hyaluronan Injections Versus Extracorporeal Shock Wave Therapy in the Treatment of Painful Achilles’ Tendinopathy: A Randomized Clinical Efficacy and Safety Study. Arch. Phys. Med. Rehabil. 2017, 98, 64–71.
- Kaux, J.F.; Forthomme, B.; Goff, C.L.; Crielaard, J.M.; Croisier, J.L. Current opinions on tendinopathy. J. Sports Sci. Med. 2011, 10, 238–253.
- Muneta, T.; Koga, H.; Ju, Y.J.; Mochizuki, T.; Sekiya, I. Hyaluronan injection therapy for athletic patients with patellar tendinopathy. J. Orthop. Sci. 2012, 17, 425–431.
- Fogli, M.; Giordan, N.; Mazzoni, G. Efficacy and safety of hyaluronic acid (500–730 kDa) Ultrasound-guided injections on painful tendinopathies: A prospective, open label, clinical study. Muscles Ligaments Tendons J. 2017, 7, 388–395.
- Kaux, J.; Bornheim, S.; Dardenne, N.; Deroisy, R.; Samson, A.J.; Roberjot, M.; Croisier, J.L. Comparison between platelet-rich plasma injections and hyaluronic acid injections in the treatment of patellar tendinopathies: A randomized trial. Muscles Ligaments Tendons J. 2019, 09, 156.
- Vora, A.M.; Myerson, M.S.; Oliva, F.; Maffulli, N. Tendinopathy of the main body of the Achilles tendon. Foot Ankle Clin. 2005, 10, 293–308.
- Gervasi, M.; Barbieri, E.; Capparucci, I.; Annibalini, G.; Sisti, D.; Amatori, S.; Carrabs, V.; Valli, G.; Donati Zeppa, S.; Rocchi, M.B.L.; et al. Treatment of Achilles Tendinopathy in Recreational Runners with Peritendinous Hyaluronic Acid Injections: A Viscoelastometric, Functional, and Biochemical Pilot Study. J. Clin. Med. 2021, 10, 1397.
- Coari, G.P.F.; Tajana, G. Fisiopatologia tendinea & Acido Ialuronico. Med. Sport. 2001, 1, 79.
- Verhaar, J.A. Tennis elbow. Anatomical, epidemiological and therapeutic aspects. Int. Orthop. 1994, 18, 263–267.
- Petrella, R.J.; Cogliano, A.; Decaria, J.; Mohamed, N.; Lee, R. Management of Tennis Elbow with sodium hyaluronate periarticular injections. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2010, 2, 4.
- Khan, I.U.; Awan, A.S.; Khan, A.S.; Marwat, I.; Meraj, M. Efficacy Of A Single-Injection Sodium Hyaluronate Treatment In Lateral Epicondylitis. J. Ayub Med. Coll. Abbottabad 2018, 30, 85–89.
- Tosun, H.B.; Gumustas, S.; Agir, I.; Uludag, A.; Serbest, S.; Pepele, D.; Ertem, K. Comparison of the effects of sodium hyaluronate-chondroitin sulphate and corticosteroid in the treatment of lateral epicondylitis: A prospective randomized trial. J. Orthop. Sci. 2015, 20, 837–843.
