2-OHM treatment induced ROS production in arabidopsis, whereas melatonin did not. ROS production by 2-OHM treatment occurred in old arabidopsis leaves in darkness, consistent with an ethylene-mediated senescence mechanism. Transgenic tobacco plants containing overexpressed rice M2H exhibited dwarfism and leaf necrosis of the upper leaves and early senescence of the lower leaves. We also demonstrated that 2-OHM-mediated ROS production is respiratory burst NADPH oxidase (RBOH)-dependent and that 2-OHM-induced senescence genes require ethylene and the abscisic acid (ABA) signaling pathway in arabidopsis. In contrast to melatonin, 2-OHM treatment induced senescence symptoms such as leaf chlorosis and increased ion leakage in arabidopsis. Senescence induction is known to begin with decreased levels of proteins involved in chloroplast maintenance including Lhcb1 and ClpR1. Together, these results show that 2-OHM acts as a senescence-inducing factor by inducing ROS production in plants
1. Introduction
In plants, melatonin is a multifunctional molecule that displays a diverse set of physiological functions in plant growth and development ranging from seed germination to seed longevity and post-harvest preservation
[1][2][3][4]. Melatonin also confers great ecological benefits when plants are challenged with adverse conditions, including diverse biotic and abiotic stresses
[5][6]. The mechanisms by which melatonin plays these physiological roles are closely dependent on its intrinsic antioxidant activity and its function as a signaling molecule in association with its receptor and downstream signaling cascades
[7][8].
In both animals and plants, melatonin is synthesized from tryptophan in a process requiring four enzymes
[9]. The last two steps of this process are well conserved in all organisms, comprising serotonin
N-acetyltransferase (SNAT) and
N-acetylserotonin
O-methyltransferase (ASMT), in biosynthesis order. Under certain conditions, this order is reversed by producing 5-methoxytryptamine through ASMT, followed by melatonin synthesis through SNAT
[10]. In contrast to conserved melatonin biosynthesis, melatonin catabolism differs greatly between animals and plants, mainly in that it is almost an end product in animals, but a precursor for further metabolites in plants. These melatonin metabolites are 2-hydroxymelatonin (2-OHM) and cyclic 3-hydroxymelatonin (3-OHM), although both are also produced nonenzymatically in animals as degradation products
[11]. In contrast, both 2-OHM and 3-OHM are predominantly and enzymatically produced in plants. Thus, these metabolites may possess their own functions in plants. In support of this hypothesis, 2-OHM is produced at a rate 300-fold higher than that of melatonin in plants
[12], and exogenously treated melatonin is rapidly converted into 2-OHM and 3-OHM by melatonin 2-hydroxylase (M2H) and melatonin 3-hydroxylase (M3H), respectively, in rice seedlings
[13]. The rapid and efficient conversion of melatonin into either 2-OHM or 3-OHM raises the question of whether the functions or phenotypes of plants treated with exogenous melatonin result from either melatonin, 2-OHM, or 3-OHM alone, or some combination of these compounds.
Previous studies have produced inconsistent reports on the effects of melatonin treatment, especially on reactive oxygen species (ROS) production in plants. Because melatonin is an antioxidant signaling molecule, its treatment does not alter ROS levels in healthy control plants and can significantly decrease ROS levels in plants challenged with many stresses
[14], as has been shown in wheat
[15] and cucumber
[16] stressed with heavy metals. In marked contrast, exogenous melatonin treatment increases ROS levels in healthy control plants but significantly decreases ROS under stress conditions such as cold
[17] or salt
[18]. However, another study found that melatonin treatment did not alter ROS levels in control plants but induced more ROS than the control under salinity stress
[19]. Surprisingly, endogenous melatonin synthesis was recently reported to closely parallel ROS production in arabidopsis, where it modulates diurnal stomatal closure
[20]. ROS production in response to melatonin treatment is regulated by respiratory burst NADPH oxidases (RBOHs)
[19][20][21][22]. Together, these findings indicate that melatonin acts as both an antioxidant and a pro-oxidant in plants, as it does in animals
[23].
2. Generation and Characterization of Transgenic Tobacco Overexpressing Rice M2H
Previously, we attempted to generate transgenic rice overexpressing the rice
M2H gene (
OsM2H) but failed because embryogenic transgenic calli were necrotized during the regeneration process, leading to lethality during somatic embryogenesis
[24]. In this study, we attempted to generate
M2H overexpression plants through organogenesis regeneration using a tobacco transformation system. We successfully generated transgenic tobacco plants without the hindrance of transgenic tobacco callus organogenesis. From 14 independent T
1 transgenic tobacco plants, we further selected three homozygous tobacco plants. These T
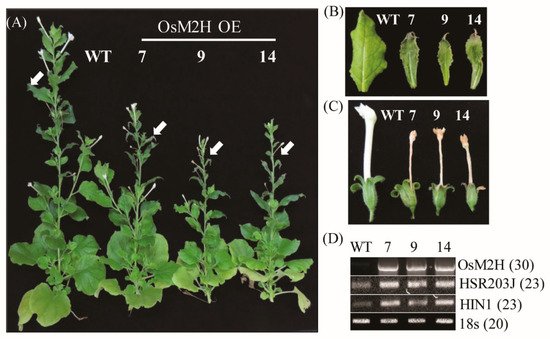
2 tobacco transgenic plants were grown to maturity (12 weeks) and showed a retarded growth phenotype compared to the wild type (WT) (
Figure 1A). The upper leaves of transgenic tobacco also showed necrotized and cell-death phenotypes in conjunction with senesced flowers compared with the WT (
Figure 1B,C). Notably, the flowers of the transgenic tobacco were smaller, with shorter corolla tubes than those of WT, but their necrotic corollae were still attached to the receptacle until the later stages of flower development. Several cell death marker genes, such as hypersensitivity-related gene (
HSR203J) and harpin-induced 1 (
HIN1)
[25], were dramatically induced in these transgenic upper leaves compared to those of the WT (
Figure 1D). The lower leaves of tobacco showed more advanced senescence in the transgenic plants than in the WT. Thus,
M2H overexpression clearly resulted in premature senescence or leaf necrosis.
Figure 1. Overexpression of OsM2H caused dwarfism and spontaneous cell death in transgenic tobacco (Nicotiana benthamiana). (A) Phenotypes of OsM2H overexpression (OE) in tobacco. Tobacco plants grown in soil under long-day growth conditions (16 h light/8 h dark) at 28 °C for up to 12 weeks were photographed. (B) Phenotypes of upper tobacco leaves, indicated by arrows in (A). (C) Flower phenotypes. (D) Reverse-transcription polymerase chain reaction (RT-PCR) analysis results. The GenBank accession numbers of OsM2H, HSR203J, and HIN1 are AK119413, AB091430, and Y07563, respectively. Numbers in parentheses indicate the number of PCR cycles.
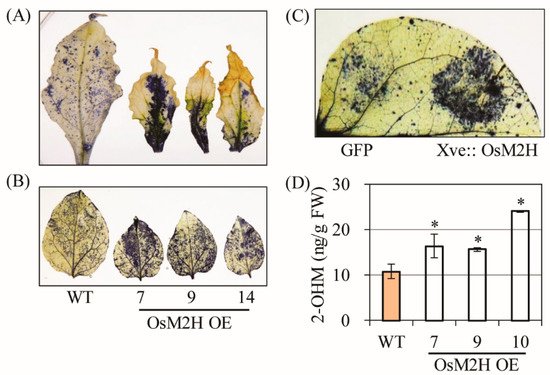
All necrotic leaves had greatly enhanced superoxide levels compared with the WT according to our NBT staining results (
Figure 2A), and young (6 weeks) tobacco leaves not showing senescence symptoms also exhibited higher superoxide levels in
OsM2H transgenic tobacco than in the WT (
Figure 2B). To determine whether ROS production was directly coupled with the
OsM2H gene in these plants, we infiltrated
Agrobacterium strains harboring
OsM2H under the control of the estrogen-inducible XVE promoter. Upon β-estradiol induction for 12 h, transient
OsM2H expression led to an increase in ROS production compared to the control green fluorescent protein (
GFP) gene, suggesting that ROS production occurred not only in transgenic tobacco expressing
M2H constitutively, but also in tobacco leaves expressing
M2H transiently (
Figure 2C). Consistent with the close relationship between
M2H and ROS production, 2-OHM content was higher in the
OsM2H transgenic tobacco leaves than in the corresponding WT (
Figure 2D). Together, these data clearly suggest that 2-OHM, the enzymatic product of the M2H enzyme, plays a direct role in ROS production and is responsible for the premature senescence of
OsM2H transgenic tobacco plants.
Figure 2. (A) Determination of superoxide levels by nitrotetrazolium blue (NBT) staining in upper leaves from 12-week-old transgenic tobacco plants. (B) Determination of superoxide levels by NBT staining in 6-week-old lower transgenic tobacco leaves. (C) Determination of superoxide levels by infiltrating Agrobacterium strains harboring the XVE-inducible OsM2H-Cherry or 35 s-GFP-HA plasmids. Six-week-old wild-type (WT) tobacco leaves were induced by estradiol (10 µM) treatment, followed by 10 h of incubation prior to NBT staining. (D) High-performance liquid chromatography (HPLC) quantification of 2-OHM in upper leaves of tobacco (Figure 1B). Error bars indicate the standard deviation of three biological replicates. Asterisks (*) indicate significant differences from the WT control, determined using Tukey’s post-hoc honest significant difference (HSD) test at a level of p < 0.05.
3. Production of Superoxide upon 2-OHM Treatment in Arabidopsis Leaves
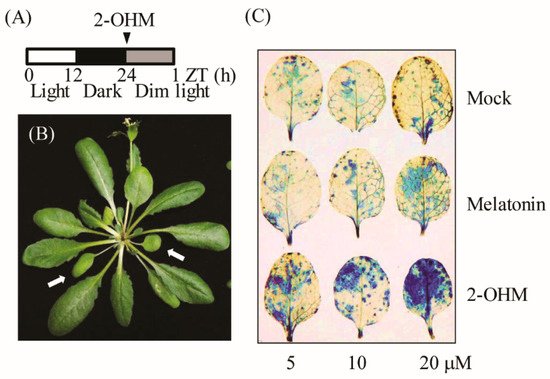
To elucidate the direct relationship between 2-OHM and ROS, varying concentrations of 2-OHM or melatonin were independently infiltrated into first and second arabidopsis leaves and incubated for 60 min under dim light conditions (7 μmol m
−2 s
−1). Superoxide levels were visualized by NBT staining. Dense NBT staining was observed in arabidopsis leaves treated with 2-OHM in a dose-dependent manner (
Figure 3), whereas mock treatments showed no visible staining (
Figure 3C). Melatonin treatment at a concentration of 20 μM resulted in slight staining. These data clearly demonstrate that ROS was mainly produced by 2-OHM, rather than melatonin, indicating that 2-OHM is the key molecule involved in ROS production. The low ROS production in response to melatonin treatment may be attributed to the conversion of melatonin into 2-OHM in arabidopsis leaves. ROS production in response to 2-OHM was barely observed in either young or rapidly growing arabidopsis leaves or old arabidopsis leaves under normal light conditions (50 μmol m
−2 s
−1), suggesting that 2-OHM is involved in senescence-related ROS production. These findings are consistent with previous reports that senescence is age-dependent and inhibited by light
[26][27][28].
Figure 3. (A) Schematic of 2-OHM treatment in arabidopsis leaves under dim light conditions (7 µmol m−2 s−1). (B) Arabidopsis leaves (arrows) grown for 6 weeks (first or second leaves) were used for 2-OHM treatment. (C) Determination of superoxide levels by NBT staining in response to varying levels of 2-OHM and melatonin. Mock treatment consisted of 5 mM MgCl2 in MES (2 mM, pH 5.6).
4. Differences in Gene Expression Patterns Elicited by Exogenous 2-OHM Treatment and Exogenous Melatonin Treatment
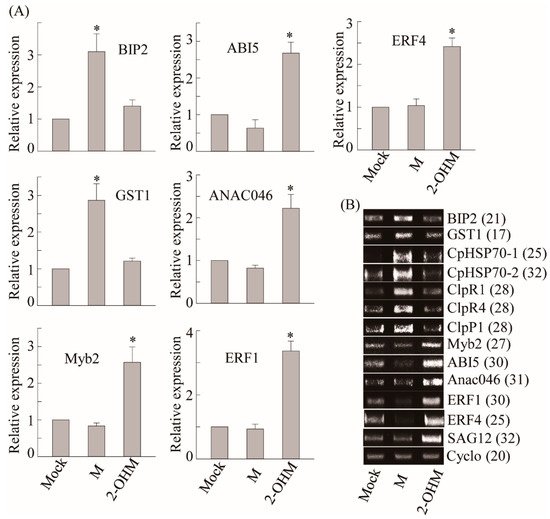
To compare differential gene expression patterns between plants treated with melatonin and 2-OHM, we selected a series of melatonin-induced genes including ROS defense-related genes such as
GST1 and protein homeostasis-related genes such as heat shock protein (
CpHSP70) and caseinolytic protease (
Clp)
[29]. Exogenous melatonin treatment (1 μM) induced a number of genes including
GST1, BIP2, CpHSP70-1, CpHSP70-2, ClpR1, ClpR4, and
ClpP1, as described previously (
Figure 4)
[29]. However, no genes induced by melatonin treatment were induced by 2-OHM treatment (1 μM), indicating the distinctive signaling roles of 2-OHM and melatonin. Based on ROS generation by 2-OHM, we monitored an array of genes involved in cell death and senescence that are associated with ethylene and ABA. The mRNA expression levels of ABA-insensitive 5 (
ABI5), MYB domain protein 2 (
Myb2), and NAC domain-containing protein 46 (
ANAC046), which are major transcription factors involved in ABA signaling, were greatly enhanced in response to 2-OHM. In contrast, these genes were downregulated by melatonin treatment. Two ethylene response transcription factors,
ERF1 and
ERF4, showed higher mRNA levels following 2-OHM treatment, but were suppressed by melatonin treatment. The expression levels of senescence-associated gene 12 (
SAG12), which is a representative senescence marker gene, were also greatly induced by 2-OHM, whereas no such induction was observed in response to melatonin. Based on these findings, 2-OHM is clearly a positive factor in senescence, whereas melatonin is a closely associated negative factor in senescence
[29]. Although 2-OHM is a simple melatonin derivative, it played completely different signaling roles than melatonin.
Figure 4. (A) Real-time PCR (qRT-PCR) analysis results for various genes involved in cell homeostasis and ABA and ET signaling in arabidopsis leaves in response to melatonin or 2-OHM treatment. (B) RT-PCR analysis results. Some genes analyzed by qRT-PCR were further analyzed by RT-PCR for clarification. Six-week-old Col-0 WT arabidopsis leaves (first or second leaves) were infiltrated with either melatonin (M; 1 μM) or 2-OHM (1 μM) and then incubated under dim light conditions (7 µmol m−2 s−1) for 2 h before leaves were collected. Mock treatment consisted of 5 mM MgCl2 (2 mM MES, pH 5.6). Expression levels were normalized to Cyclo for qRT-PCR. Asterisks indicate significant differences from the mock control as determined by Tukey’s post-hoc HSD test at a level of p < 0.05. GenBank accession numbers were as follows: BIP2 BIP2 (AT5g42020), GST1 GST1 (AT1g02920), CpHSP70.1 CpHSP70.1 (AT4g24280), CpHSP70.2 CpHSP70.2 (AT5g49910), ClpR1 ClpR1 (AT1g49970), ClpR4 ClpR4 (AT4g17040), ClpP1 ClpP1 (ATCg00670), Myb2 Myb2 (AT2g47190), ABI5 ABI5 (AT2g36270), Anac046 Anac046 (AT3g04060), ERF1 ERF1 (AT3g23240), ERF4 ERF4 (AT3g15210), SAG12 SAG12 (AT5g45890), and Cyclo Cyclo (AT4g38740). Numbers in parentheses indicate the number of PCR cycles.
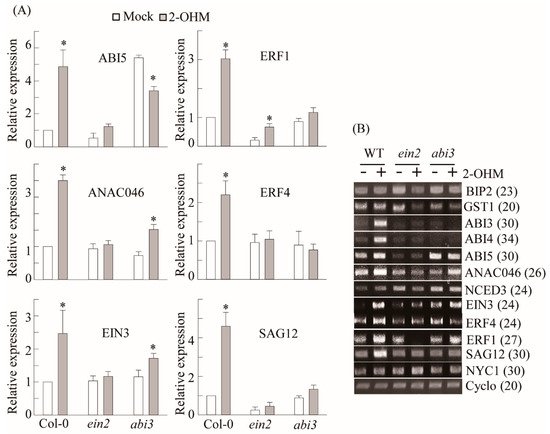
5. Ethylene and ABA Signaling Was Required for 2-OHM-Induced Gene Expression
To determine whether 2-OHM-mediated gene induction of ethylene and ABA-related transcription factors is dependent on ethylene and ABA signaling, we employed knockout mutant lines of
EIN2 and
ABI3, which are key signaling factors of ethylene and ABA
[30]. Ethylene-related transcription factors including
EIN3,
ERF1, and
ERF4, which were induced by 2-OHM treatment, were abolished in the
ein2 mutant (
Figure 5). Similarly, ABA-related transcription factors such as
ABI3,
ABI4, and
ABI5 failed to be induced by 2-OHM in the
abi3 mutant and
NCED3, an ABA biosynthetic gene that encodes 9-cis-epoxycarotenoid dioxygenase 3, was slightly increased upon 2-OHM treatment, whereas this induction was reversed slightly in the
abi3 mutant. The induction of
ANAC046, an NAC transcription factor and positive regulator of chlorophyll degradation, was abolished in the
ein3 mutant, whereas its expression was significantly reduced in the
abi3 mutant. In contrast, NON-YELLOW COLORING 1 (
NYC1), which is involved in chlorophyll degradation, was not responsive to 2-OHM treatment. These data suggest that 2-OHM plays more important roles in ethylene and ABA signaling cascades than in their biosynthetic pathways.
Figure 5. EIN2- and ABI3-dependent gene induction in response to 2-OHM treatment. (A) qRT-PCR analysis results. (B) RT-PCR analysis results. Arabidopsis WT (Col-0), ein2, and abi3 plants were treated with 2-OHM (1 µM). Leaves were infiltrated with solutions and incubated under dim light conditions for 2 h prior to sample harvesting. Mock treatment consisted of 5 mM MgCl2 in 2 mM MES (pH 5.6). Cyclo was used for qRT-PCR normalization and as a loading control for RT-PCR. GenBank accession numbers were as follows: ABI3 (AT3g24650), ABI4 (AT2g40220), NCED3 (AT3g14440), EIN3 (AT3g20770), and NYC1 (AT4g13250). Other genes are listed in Figure 4. Asterisks (*) indicate significant differences from the mock control, determined using Tukey’s post-hoc HSD test at a level of p < 0.05. Numbers in parentheses indicate the number of PCR cycles.
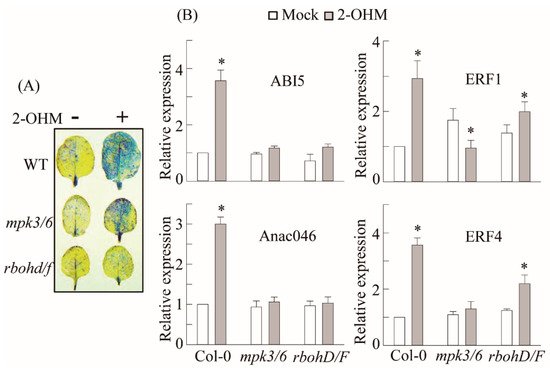
6. RBOH-Dependent ROS Production and Ethylene and ABA Signaling Requirement of 2-OHM
In plants, ROS are generated by either RBOH located in the plasma membrane or photo-activated chloroplasts. Some studies have suggested that melatonin may induce RBOH in plants
[19][21]. Many melatonin-mediated defense responses against pathogen
[31], high-light
[32], low-light
[29], and ER
[33] stress are mediated by the mitogen-activated protein kinase (MPK) pathway. Therefore, we examined the possible involvement of RBOH and MPK3/6 in 2-OHM-mediated ROS production as well as ethylene and ABA signaling in arabidopsis. First, we measured superoxide production following 2-OHM treatment in either the
rbohD/F double knockout mutant or the
mpk3/6 double RNAi line. Superoxide production following 2-OHM treatment was completely arrested in the
rbohD/F mutant, but not in the
mpk3/6 RNAi line, when compared to WT Col-0, suggesting the absolute dependency of 2-OHM-mediated ROS production on RBOH, but not MPK3/6 (
Figure 6). Next, we monitored gene expression levels associated with ethylene and ABA signaling. All of these genes were barely induced in the
rhohD/F and
mpk3/6 lines in response to 2-OHM treatment (
Figure 6B), indicating the strong dependence of 2-OHM-mediated ethylene and the ABA signaling pathways on both RBOH and MPK3/6. Although MPK3/6 is not essential for ROS generation by 2-OHM, MPK3/6 is critical for the induction of ABA and ethylene-related transcription factors. These data indicate that both melatonin and 2-OHM accept the MPK pathway as an integrated mediator to activate their own distinctive signaling. These findings also suggest that the ROS RBOHD/F acts upstream of MPK3/6 signaling when arabidopsis leaves are exogenously treated with 2-OHM.
Figure 6. Involvement of MPK3/6 and RHOHD/F in 2-OHM-mediated superoxide and senescence-related gene induction. (A) Determination of superoxide levels by NBT staining. (B) qRT-PCR analysis results for ethylene and ABA signaling-related genes. Six-week-old WT, mpk3/6, and rbohD/F leaves were treated with 2-OHM (1 µM) as described for Figure 3. (*)Asterisks indicate significant differences from the mock control, as determined by Tukey’s post-hoc HSD test, at a level of p < 0.05.
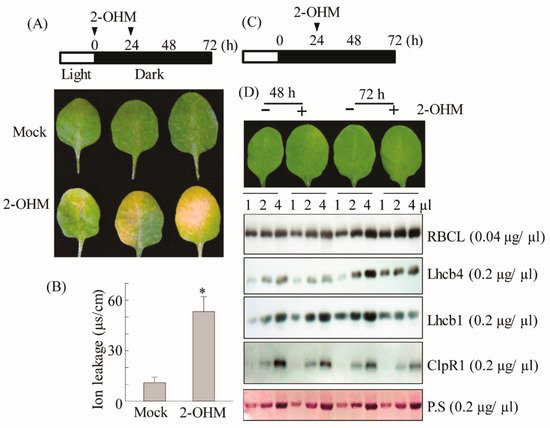
7. Acceleration of Dark-Induced Senescence upon 2-OHM Treatment in Arabidopsis
Based on ROS production and ethylene and ABA signaling gene induction by 2-OHM, we hypothesized that 2-OHM could be a senescence-inducing factor. To test this hypothesis, the first or second arabidopsis leaves of 6-week-old plants were infiltrated abaxially with 10 μM 2-OHM twice (at 0 and 24 h), followed by incubation in the dark to monitor darkness-induced senescence symptoms. We found that 2-OHM triggered clear leaf chlorosis and increased ion leakage levels (
Figure 7A,B); these symptoms were not observed under light or in young or mature arabidopsis leaves (data not shown), suggesting that 2-OHM does not act as a senescence-inducing signal under daylight conditions or in young leaves. Because M2H protein is localized in chloroplasts
[34], 2-OHM is likely first produced within chloroplasts. To determine whether 2-OHM affects chloroplast function, we monitored the expression levels of the light-harvesting antenna protein Lhcb1 and the key chloroplast molecular chaperone ClpR1 (caseinolytic protease) in response to 2-OHM treatment under dark incubation. The Lhcb1 level affects chlorophyll levels and state transition in arabidopsis
[35], and ClpR1 is essential for chloroplast maintenance by controlling Lhcb2 protein levels, such that the ClpR1-knockout mutant results in an abnormal arabidopsis phenotype
[36]. We administered one treatment of 2-OHM (10 μM) at 24 h after a dark incubation period (
Figure 7C) to avoid the severe senescence symptoms shown in
Figure 7A. Leaves harvested at 48 and 72 h after dark incubation were assayed for protein levels. The protein levels of ribulose-1,5-bisphosphate carboxylase/oxygenase (RBC) large subunit (RBCL) were not affected in 2-OHM-treated leaves compared with the mock control leaves. However, the expression levels of Lhcb1, Lhcb4, and ClpR1 proteins were reduced in leaves treated with 2-OHM compared with those in the mock control. These results indicate that 2-OHM treatment induces protein instability of chloroplast maintenance components, eventually leading to rapid necrotic chlorosis under darkness-induced senescence. In marked contrast, the opposite results were achieved by melatonin treatment of arabidopsis leaves, which increased the expression levels of Lhcb1, Lhcb4, and ClpR1 proteins
[29].
Figure 7. (A) Schematic diagram of 2-OHM treatment for senescence induction and the resulting phenotypes. (B) Ion leakage measurement. (C) Schematic diagram of 2-OHM treatment for physiological tests. (D) Western blot analysis using anti-RBCL, -Lhcb4, -Lhcb1, and -ClpR1 antibodies in the WT in response to 2-OHM treatment. We administered 2-OHM (10 µM) into Col-0 leaves twice (0 and 24 h) as described for Figure 3. Leaves were collected at 72 h for photography (A) and ion leakage measurements (B). A single treatment of 2-OHM was administered at 24 h to measure protein levels involved in chloroplast homeostasis. Total leaf protein extracts were subjected to 14% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The immunoblot was probed with specific antibodies (right). Bottom panel shows a loading control stained with Ponceau S solution (P.S). Mock treatment consisted of 5 mM MgCl2 in 2 mM MES (pH 5.6). (*) Asterisks indicate significant differences from the mock control, determined by Tukey’s post-hoc HSD test at a level of p < 0.05.