Due to its piezoelectric, ferroelectric, nonlinear optics, and pyroelectric properties, LiNbO3 crystal has found its wide applications in surface acoustic wave (SAW) devices, optical waveguides, optical modulators, and second-harmonic generators (SHG). LiNbO3 crystallized as R3c space group below Curie temperature shows spontaneous polarization that leads to its ferroelectric and piezoelectric properties. Physical and chemical characteristics of LiNbO3 are mainly determined by Li/Nb ratio, impurity cations, vacancies in a cation sublattice. Different sizes of LiNbO3 ranging from nanoscale and microscale to bulk size have been synthesized by solid state method, hydrothermal/solvothermal method, Czochralski (Cz) growth method, etc. Most basic and applied studies of LiNbO3 focus on its bulk single crystal.
- LiNbO3
- crystal growth
- piezoelectric property
- optical property
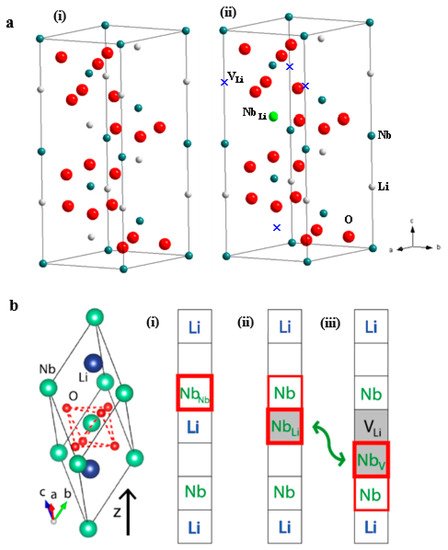
1. Crystal and Defect Structures of LiNbO3
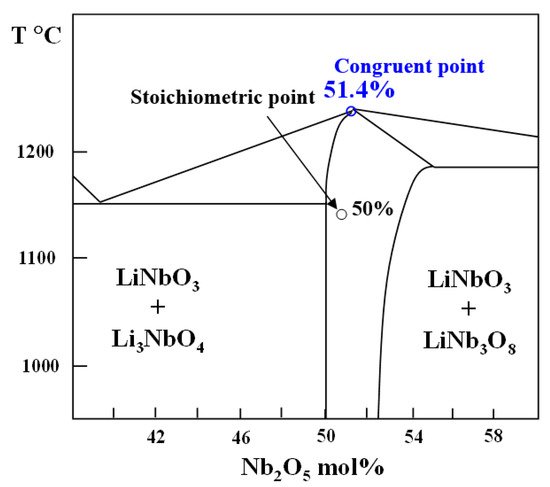
2. Crystallization of LiNbO3
| Testing Method | Advantages | Disadvantages | ||
|---|---|---|---|---|
| Raman scattering method | Raman systems have become cheaper and easier to use | The use of a correct configuration of the detection and excitation polarizers (in the case of single crystals) | ||
| Curie temperature | Linearly with Li/[Li + Nb] ratio Reliable and sufficient sensitivity for composition |
High Curie temperature close to the melting point | ||
| UV absorption edge | Convenient and accurate way for determining the composition | Nonlinear relationship Accuracy is governed by the wavelength calibration Doping compound will deteriorate the accuracy | - | [14][28] |
| 0.4–1.5 | 10–30 | 50 × 30 | Δ[Li2O] < 0.005 | [15][29] |
| 1–2.5 | 10–25 | 80 × 60 | Δ[Li2O] < 0.02 | [16][30] |
| 2.8–4.0 | 3–10 | 100 × 80 | Δ[Li2O] < 0.002 | [8][22] |
3. Composition Characterizations of LiNbO3
| Refractive indices | ||
| Function of wavelength and stoichiometry | Nonlinear relationship | |
| Birefringence | Approximately linear correspondence between Li content and birefringence | The nonlinear relationships dominated by the wavelength |