Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Md. Habibur Rahman and Version 3 by Yvaine Wei.
Curcumin is the primary polyphenol in turmeric’s curcuminoid class. It has a wide range of therapeutic applications, such as anti-inflammatory, antioxidant, antidiabetic, hepatoprotective, antibacterial, and anticancer effects against various cancers, but has poor solubility and low bioavailability.
- nanocurcumin
- solubility
- antioxidant effect
- anticancer
- neurodegenerative disease
1. Introduction
Curcumin is a bioactive compound and is the active component of Curcuma longa (C. longa), which is turmeric, a member of the ginger family. It is used as a spice, culinary coloring, and a component in ancient herbalism. Curcumin, a polyphenol, has been demonstrated to target various signaling molecules while also displaying cellular activity, contributing to its multiple health advantages. It also serves as an antioxidant, anti-inflammatory, and anticancer agent. Curcumin has been proven to have antioxidant, anti-inflammatory and anticancer effects and the ability to enhance cognitive skills and manage obesity and diabetes [1]. In Asian countries, C. longa has long been used as a prescription or supplement to treat diabetes, coronary disease, obesity, neurodegenerative disease, inflammatory bowel disease, allergy or asthma, and psoriasis [2]. C. longa is grown in tropical and subtropical climates. India is the world’s largest producer of turmeric, which has long been used as a home cure for various diseases [3]. Even though the extraction and isolation of curcumin from turmeric powder was first published in 1815, newer and more advanced extraction methods are still reported two centuries later [4]. The most frequent method for separating curcumin from turmeric has been solvent extraction followed by column chromatography, and numerous polar and nonpolar organic solvents have been utilized, including hexane, ethyl acetate, acetone, methanol, and others. For extracting curcumin, ethanol was determined to be the most preferred solvent among the organic solvents used. Although chlorinated solvents extract curcumin from turmeric quite effectively, they are not widely used in the food business because of their unacceptability. Soxhlet extraction, ultrasonic extraction, microwave extraction, zone refining, and dipping procedures have been tried, with the most being popular Soxhlet, ultrasonic, and microwave extractions [5]. Curcumin is insoluble in water; thus, it was isolated using an organic solvent. The source and chemical structure of curcumin are given in Figure 1.
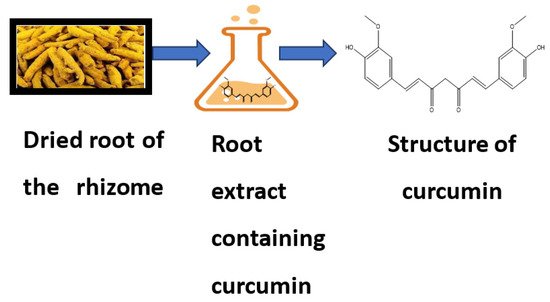
Figure 1.
Source and chemical structure of curcumin.
2. Bioavailability of Curcumin
Despite numerous health benefits, curcumin’s limited bioavailability is a fundamental criticism [6][10]. Low absorption, fast metabolism, chemical instability, and rapid systemic clearance have been suggested as possible causes [7][11]. According to various animal studies, most oral curcumin is eliminated in the feces (90%). Several strategies have been tried to boost the bioavailability of curcumin to solve this problem. Piperine, liposomal curcumin, curcumin nanoparticles, phospholipid complexes, and structural analogs of curcumin such as turmeric oil are a few examples of adjuvants [8][12]. Increased blood concentrations have been observed as a result of such efforts. However, human clinical trials comparing the therapeutic potencies and pharmacodynamic responses of these more bioavailable variants to those of conventional curcumin have yet to be undertaken extensively. Furthermore, the serum concentrations required to achieve a particular clinical or biological effect have yet to be determined. Piperine is an alkaloid found mainly in Piper nigrum, and it is this component provides black pepper its piquancy. Piperine has been shown to increase curcumin bioavailability [8][12]. Piperine suppresses the liver enzyme UDP-glucuronyl transferase, reducing the amount of glucuronidation of curcumin. Additionally, curcumin is accessible for ingestion as a result of this mechanism [9][13].
Nanocarrier-based delivery of curcumins one of the best approaches to improve curcumin’s solubility and bioavailability while also protecting it from hydrolysis-induced inactivation. Some nanocarriers emphasized long-term retention and circulation in the body, while others focused on intracellular release mechanisms and cellular delivery. Curcumin is solubilized in these environments by becoming entrapped in hydrophobic pockets, primarily through hydrophobic interactions. Curcumin’s fluorescence is boosted when solubilized in any of these systems, making it simple to measure its binding effectiveness. Because of their biocompatibility, these systems may be successfully studied for anticancer activity in cancer cells and in vivo systems, with considerable increases in anticancer activity due to enhanced curcumin bioavailability reported. Curcumin liposomal formulations have been proven to be the most effective for increasing curcumin bioavailability in cells [10][19], and products based on liposomal formulations are being commercialized. Nanocurcumin’s effectiveness is due to the size, surface area, charge, and hydrophobic nature of the particles, which make it superior to native curcumin [11][20] and regarded as an acceptable target for usage as a drug compared to standard curcumin. This feature is especially crucial in the fight against infectious illnesses caused by intracellular infections [12][21]. According to Ma et al. [13][22], nanocurcumin increased in vivo bioavailability and tissue distribution, with a 60-fold increase in biological half-life compared to native curcumin treatment in rat models.
3. Therapeutic Applications of Nanocurcumin over Curcumin
Nanocurcumin has the potential to prevent and treat a wide range of human diseases.3.1. Antioxidant Effects
The antioxidant property of curcumin is due to its structure, which interconnects two methoxylated phenols with two unsaturated carbonyl groups grouped in stable enol form [14][23]. The phenolic O-H and C-H groups in curcumin are responsible for its bioactivity. Curcumin also plays a role in lipid peroxidation inhibition by oxidizing a polyunsaturated fatty acid called linoleate, which is oxidized to generate a fatty acid radical. Curcumin also participates in the intermolecular Diels–Alder reaction by breaking the chain at the 3’ site and neutralizing lipid radicals [15][24]. Besides lipid peroxidation inhibition, curcumin has been shown to have free radical scavenging activities in in vitro and in vivo models employing rat peritoneal macrophages. Curcumin actively scavenges numerous reactive oxygen species (ROS) produced by macrophages, such as hydrogen peroxide, nitrite radicals, and superoxide anions [16][25].3.2. Anti-Inflammatory Effects
Curcumin aids in the battle against invaders and aids in the healing of the rift zone. While acute, short-term inflammation is helpful, it may become a problem if it becomes persistent and destroys cells. Excessive inflammatory activation for long periods of time can result in mitochondrial dysfunction. Traumatic brain injuries (TBI), spinal cord injuries (SCI), and hemorrhagic/ischemic stroke all cause substantial changes in mitochondrial dynamics, notably increased membrane permeabilization, oxidative phosphorylation, and the accumulation of mitochondrial ROS [17][18][31,32]. Excessive glial activation has been linked to the same outcomes as long-term inflammation. Advanced mitochondrial dysfunction has also been demonstrated to increase inflammatory processes, leading in neuronal injury and poor neurological consequences [19][20][33,34]. Studies have identified that the gene CDGSH iron sulfur domain 2(CISD2) protects against inflammatory reactions and apoptosis caused by mitochondrial dysfunction. The presence of CISD2 in the outer membrane of mitochondria has also been linked to the preservation of mitochondrial integrity. CISD2 deficiency resulted in mitochondrial dysfunction and cell death, according to reports on CISD2 knockout mice [21][22][35,36]. The attachment of BCL2 to BECN1, which governs cellular autophagy/apoptosis, has been found to be aided by CISD2. Anti-inflammatory and/or antiapoptotic therapeutics based on CISD2 are extremely likely to be developed to combat the consequences of aging, neurodegenerative disease, and CNS trauma [23][37].3.3. Antibacterial Effects
The dramatic rise in microbe resistance to multiple medications has necessitate daquest for novel and potentially effective antibacterial agents with minimal or lower human cytotoxic effects that can aid in treating a wide range of microbial diseases [24][46]. Curcumin antibacterial effectiveness against Gram-positive bacteria (Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli) was studied by Tyagi et al. [25][47]. Curcumin was shown to have good antibacterial activity against all tested microorganisms [26][48]. Curcumin’s increased antibacterial action in the form of sunlight radiation mainly owed to photoexcitation, which caused the production of ROS that inhibited bacteria load [26][48].4. Various Nano Drug Delivery Systems for Curcumin
4.1. Liposomes
Natural phospholipids can be used to produce liposomes, which are globular synthetic vesicles. They are inner enclosed colloidal materials made up of lipid bilayers with an exterior lipid bilayer around a core aquatic region [27][28][114,115]. Liposomes range in size from 25 to 205 nm in diameter. Liposomes are supposed to be utilized as drug transporters and immunological assistance agents and are encapsulated either in the aquatic part of the lipid bilayers or at the bilayer surface. They can be used to encapsulate drugs with a wide range of solubilities or lipophilicities [29][30][116,117]. Furthermore, they can carry drugs into cells by fusion or endocytosis, and almost any chemical, regardless of solubility, can be encapsulated in liposomes (Figure 2). To enhance curcumin solubility, Rahman et al. [31][118] produced β-cyclodextrincurcumin binding interactions that incorporated both natural curcumin and the complexes independently into liposomes. Entire curcumin-containing preparations were efficient at suppressing cell growth in vitro cell study [32][119]. Shi et al. [33][120] used an enzyme-linked immunosorbent assay (ELISA) technique to evaluate curcumin’s therapeutic benefits on lung fibrosis in mice using a water-soluble liposomal curcumin that was found to successfully reduce radiation pneumonitis and lung fibrosis and sensitize LL/2 cells to irradiation. Some research has indicated that liposome-encapsulated medication is predicted to be delivered without accelerated deterioration and results in fewer adverse consequences and exhibit greater evidence of resilience in the receiver. Matabudul et al. [34][121] investigated whether various intervals of Lipocurc intravenous infusions affected metabolism and tissue delivery of curcumin, and whether reacting necropsied beagle dog tissues with phosphoric acid before evaluating curcumin and its active ingredient (tetrahydrocurcumin) could regulate the substances and allow effective assessments. Figure 2 4 illustrated various nano drug delivery system for curcumin delivery.
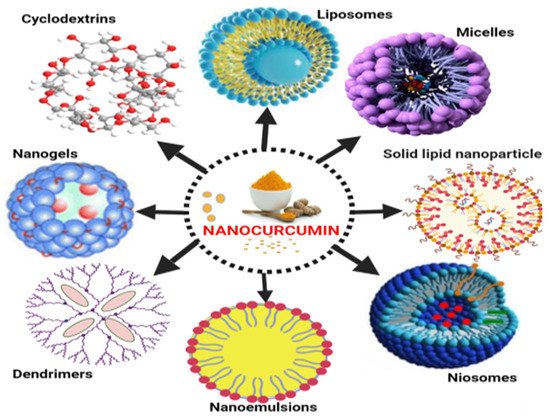

Figure 24.
Various nano drug delivery systems for curcumin.
Curcumin-loaded nanoparticles have been used to assess liposomal curcumin’s potential against various cancers [35][36][37][122,123,124]. Liposomal curcumin demonstrated significant anticancer potential against osteosarcoma and breast cancer cell lines in vitro and in vivo via the caspase cascade, which resulted in apoptotic cell death. The xenograft osteosarcoma model in vivo was used to demonstrate the efficacy of liposomal curcumin nanoparticles. Curcumin was incorporated into a liposomal delivery system for intravenous administration by Li et al. [38][125]. These authors also used human pancreatic cancer cells in vitro and in vivo to demonstrate the effects of liposome-encapsulated curcumin on proliferation, apoptosis, signaling, and angiogenesis [39][40][126,127]. Curcumin encapsulated in liposomes inhibited tumor angiogenesis in vivo and suppressed pancreatic carcinoma growth in murine xenograft models [41][128]. It also inhibited proliferation, caused apoptosis, and inhibited the NF-κB pathway in human pancreatic cells in vitro, as well as having anticancer and antiangiogenesis actions in vivo [42][43][129,130]. Curcumin partitioning into liposomes containing dimyristoyl phosphatidyl choline (DMPC) and cholesterol suppressed cellular proliferation in human prostate cancer cell lines (LNCaP and C4B2) by 70–80% without affecting viability. When compared to curcumin, oral doses of liposome-encapsulated curcumin resulted in high bioavailability and quicker and better absorption of curcumin in rats [44][131]. Curcumin is incorporated into liposomes and then reaches cells are demonstrated in Figure 35.
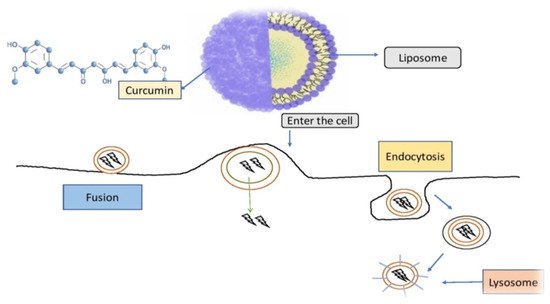

Figure 35. Curcumin is incorporated into liposomes and then reaches cells in this diagram. Curcumin is enclosed within the liposomal vessel and chemically attached to the liposome, preventing it from being destroyed on its approach to the target. Phospholipids, which are found in biological membranes and may deliver curcumin to cells via fusion and endocytosis, are frequently used in liposome membranes [45][132].
4.2. Micelles
A micelle is a spherical vesicle made up of amphiphilic surfactant molecules that spontaneously assemble in water. Micelles are commonly utilized to deliver drugs that are not very water soluble, such as curcumin [46][133]. Curcumin can be added pre- or post-micelle production to solubilize it within the hydrophobic center of micelles. Micelles are colloidal dispersions that are highly thermally stable and include particulates. Because the particles are so tiny, they do not scatter light waves substantially and thus tend to be optically transparent. Micelle viscosities are determined by surfactant concentration and micelle structure. Micelles often seem to be fluids with low viscosities at low concentrations, but semisolids with greater viscosities or gel-like textures at higher concentrations. Since these variables impact the size, shape, interactions, and dynamics of the colloidal structures produced, the rheological characteristics of micellar systems are highly influenced by the surfactant type and ambient circumstances [47][134]. Micelles improve the bioavailability of hydrophobic substances (such as curcumin) by enhancing their bioaccessibility in gastrointestinal fluids and potentially boosting epithelial cell penetration [48][135]. Curcumin encapsulated polymeric micelles (CUR-M) were produced using a one-step solid dispersion method, and the efficacy of CUR-M was tested in a breast tumor model, by Liu et al. [49][136]. CUR-M exhibited better activity than pure curcumin at suppressing the generation of breast cancers and spontaneous pulmonary metastases of the lungs. Curcumin-poly (ethylene glycol) methyl ether (MPEG-PCL) micelles with solid dispersion improved curcumin’s antiangiogenesis and antitumor effects.
