1. Licorice (Glycyrrhiza glabra)
Licorice (also called liquorice) is a Greek word meaning ‘sweet root’ and is a perennial herb native to southwestern Asia and the Mediterranean region in Europe
[1][56] (
Figure 12). The plant has been used in several locations in India, China, Greece, Europe, the Middle East, and Africa for various treatments, particularly those related to arthritis and ulcers
[2][57]. It is known as ‘Yashtimadhu’ (sweet root) in Sanskrit and ‘Gan cao’ (sweet grass) in the Chinese language
[2][57] and was also used in Arabic medicine in the Middle Ages and documented in the Canon of Ibn Sina (980–1037 AD)
[1][56]. The herb belongs to the
Glycyrrhiza glabra species in the Leguminosae family and grows to a height of around 2 m
[3][58].
Figure 12.
Licorice plant (
left
) and its root (
right
).
The plant has a long cylindrical-shaped, multi-branched root, which extends horizontally underground and is mainly used for medicinal purposes
[4][59]. Licorice is 50 times sweeter than sugar due to the presence of glycyrrhizin (glycyrrhizic acid-GL) and is often used as a sugar substitute
[4][5][59,60]. The plant has antioxidant properties; therefore, it is used in some cancer treatments
[4][6][59,61]. The licorice family has three original plants used for treatment:
G. uralensis, G. inflata, and
G. glabra [7][62]. The licorice plant is considered a weed in many places like wheat crops and cotton plantations, together with potato, sugar beet, clover, and sainfoin fields, but it has been used as traditional medicine in many ancient civilizations such as for the Greeks, Romans, Egyptians, Assyrians, Indians, and Chinese
[8][9][55,63].
Licorice falls into the list of first-line medicinal plants
[10][11][12][64,65,66]. In ancient China and India, licorice has been used for more than 5000 years to treat respiratory and liver disease and alleviate the toxicity of other drugs
[1][7][9][56,62,63]. The Ayurvedic Pharmacopoeia of India
[9][63] mentions that licorice treats inflammation, eye and liver diseases, throat infections, peptic ulcers, and arthritis. Greeks used licorice to treat both gastric and peptic ulcers, whilst Europeans and Asians have utilized it to treat psoriasis
[13][67]. It is a traditional Persian medicine to treat various diseases, including respiratory disease
[14][68]. The role of licorice in Japan also needs to be highlighted as they have been using it to treat chronic hepatitis
[14][68]. The plant also has anticancer, hepatoprotective, antispasmodic, neuroprotective, antioxidant, and estrogenic properties and is very useful in reducing hepatocellular damage in chronic hepatitis B and C patients
[15][69]. For many years, it has had the reputation of being a memory booster
[16][70] and an antidepressant
[17][71] and can reduce blood cholesterol levels
[18][19][72,73] and acts as a promising drug for treating liver and renal complications
[20][21][22][74,75,76]. It is also found that the plant can reduce polydipsia and frequent urination in diabetic patients
[15][69]. In some places, the root is used to prepare tea, and the dried root is used as a tooth cleanser
[23][77].
When studies on the properties and therapeutic benefits of phytochemicals in the licorice have been undertaken, scientists discovered that they could extract more than 20 triterpenes, 300 flavonoids, and 73 bioactive compounds from the root and identified 91 potential targets for its action
[7][24][25][62,78,79]. Many of the extracted bioactive compounds were shown to have antimicrobial, antiviral, and anti-inflammatory properties, such as GL, 18ἁ/β-Glycerrhetinic acid (GA), three triterpenes, and several flavonoids (
Table 1).
Table 1. A list of major bioactive compounds identified in licorice together with their therapeutical properties.
| Bioactive Compounds |
Properties |
References |
][98] believe that licochalcone could be used to synthesize novel anti-
S. aureus compounds that may inhibit the production of ἁ-toxin in methicillin-sensitive
S. aureus (MSSA) and MRSA. In addition to antibacterial efficacy, the antifungal efficacy of licochalcone and glabridin has been observed by Messier and Grenier
[36][90].
2.1. Antiviral Activity
In Vitro Studies
One of the significant characteristics of licorice, which may be useful for treating COVID-19, is its antimicrobial property, particularly its antiviral effect
[41][45][95,99]. The antiviral property of licorice is revealed by many researchers, with the first study being published in 1979
[46][100], whose finding was that GL and GA (structure in
Figure 3) are the main compounds behind the antiviral efficacy
[47][101]. Ashfaq et al.
[48][102] investigated the antiviral characteristic of licorice against the hepatitis C virus. They demonstrated that the plant extract could inhibit hepatitis C virus’ growth, including a 50% reduction (14 ± 2 µg/mL) in viral concentration (including assessment of the full-length particle and core gene expression). Through the experiment, Matsumoto et al.
[47][101] found that GL targets the release step of the hepatitis C viral infection, which identified a potential role for GL in hepatitis C treatment. Huang et al.
[49][103] studied the effect of GL on HIV infection and found that GL perfusion can inhibit HIV infection by reducing its adhesion and stress components. Later, the efficacy of GL against Coxsackievirus A16 (CVA16) and Enterovirus 71 (EV71) was studied by Wang et al.
[2][57]. GL’s effectiveness against the influenza virus was also studied, and it was observed that GL could inhibit the H5N1-induced production of chemokine ligand 5 (C-C motif CCL5) and ligand 10 (C-X-C motif, CXCL10), together with IL-6
[50][51][52][104,105,106]. It was also found that GL can suppress H5N1-induced apoptosis activity at a concentration of 100 µg/mL
[50][104]. Apart from this, the action of GL against the herpes simplex virus (HSV1) is studied by Laconi et al.
[53][107] and found that pre-treatment with GL on the HeLa cell improved the antiviral property (when experimented with HSV1) by a factor of 95 to 98%. Besides viral inhibition, GL also evidences immunostimulant activity against viruses such as the duck hepatitis virus (DHV)
[54][108]. Both antiviral and antitumor activity of licorice root extracts were investigated by Fukuchi et al.
[55][109], who found that the alkaline extracts demonstrated higher antiviral activity against HIV compared to the water extract. This led the authors to suggest that this extract may be converted into mass production as an anti-HIV agent.
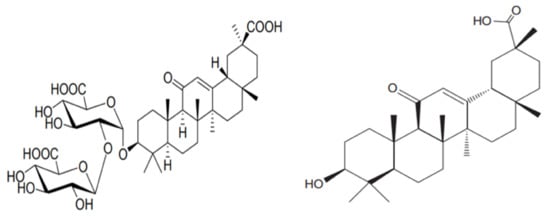
Figure 3. Chemical structure of Glycyrrhizic Acid (GL) (left) and 18β-Glycerrhitinic acid (GA) (right).
An epidemic with a severe coronavirus (SARS-CoV) started in 2002 in China and spread to 32 countries. After this epidemic, many researchers studied the efficacy of licorice, specifically GL, on SARS-CoV. The study of Hoever et al.
[56][110] is significant, and their in vitro study revealed that GL was able to inhibit virus replication. Among the 15 GL derivatives, 2-acetamido-β-D glucopyranosylamine, when inserted into the glycoside chain of GL, showed a ten-fold higher antiviral efficacy than normal GL. It was also observed that GL could inhibit the absorption and penetration of the SARS-CoV in the early replicative cycle, specifically when given during and after the absorption period. However, due to the complexity of this mechanism, the exact activity details are unclear, but there is a suggestion that nitrous oxide (NO) donation is somehow involved
[1][57][56,111].
Apart from GL, the antiviral activity of GA was also studied against many viruses, including the human respiratory syncytial virus (HRSV), arbovirus, vaccinia, and vesicular stomatitis. It was identified that both GL and GA could induce interferons that can bind to cell surfaces and stimulate the synthesis of intracellular proteins, blocking the transcription of viral DNA
[58][112]. Interferon also activates the macrophages and stimulates the augmentation of the natural killer cell activity
[58][112]. All of these studies demonstrate that GL and GA in licorice, particularly GL, can be used as a potential antiviral drug (
Table 2). Therefore, it is suggested to undertake in vitro and in vivo studies followed by clinical trials to investigate the antiviral efficiency of GL against SARS-CoV-2 replication.
Table 2. A list of significant phytochemicals present in licorice and their antiviral efficacy.
| Compounds in Licorice |
Antiviral Property against |
Reference |
| Glycyrrhizic acid |
SARS-CoV |
[57][111] |
| Glycyrrhizic acid derivatives |
SARS-CoV |
[56][110] |
| Glycyrrhizic acid |
Hepatitis A (HAV) |
[59][113] |
| Glycyrrhizic acid |
Hepatitis B (HBV) |
[27][60][61][81,114,115] |
| Glycyrrhizic acid |
Hepatitic C virus |
[47][48][62][63][101,102,116,117] |
| Glycyrrhizic acid |
Human immune deficiency (HIV) Virus |
[49][64][65][103[,11866,119],120] |
| Alkali root extract |
HIV |
[55][109] |
| Glycyrrhizic acid |
Herpes viridae (varicella) |
[67][121] |
| |
Zoster virus (VZV) |
[68][122] |
| |
Epstien-Barr virus (EBV) |
| Glycerrhizin (GL) |
Antimicrobial |
[26][27][28][80,81,82] |
| 18β-Glycerrhitinic acid |
Antimicrobial, anti-inflammatory |
|
| (GA) |
Against Helicobacter pylori, |
[29][30][31][83,84,85] |
| |
MRSA, |
|
| |
Clarithromycin-resistant H. pylori |
|
| 18α-GC, 18β-GC |
Anti-inflammatory |
|
| Flavonoids (13 Ns below) |
Anti-inflammatory |
|
Licochalcone A/B/C/D/E,
isoliquiritigenin (ISL)
echinatin (EC), glabridin (GLD),
soangustone A (ISOA),
licoricidin (LID),
licorisoflavan A (LIA),
dehydroglyasperin C (DGC), &
dehydroglyasperin D (DGD)
Glabridin |
Antimicrobial, anti-inflammatory |
[32][86] |
| Antimicrobial |
[33][87] |
| Aqueous extract |
B. Subtilis and E. Coli |
[34][88] |
| Methanol extract |
Phytopathogenic fungi |
[35][89] |
| Glycyrhetinic acid |
MRSA |
[30][84] |
| Licochalcone and Anti-fungal |
|
[36][90] |
| Acetate root extract |
K. pneumonia and A. baylyi |
[37][91] |
| Glabron |
|
|
| Licochalcone A/C/E |
Staphylococcus aureus |
[38][92] |
| Glycyrrhizin |
Helicobacter pylori |
[39][93] |
| 18β-Glycerrhetinic acid |
Clarithromycin-resistant H. pylori |
[31][85] |
The roots contain several phytochemicals such as 2β-GL, glucuronic acid, GA, tannic acid asparagine, resins, volatile oils, flavonoids such as liquiritigenin (LG), liquiritin (LQ), isoliquiritigenin, isoliquiritin, and coumarin compounds such as herniarin and umbelliferone. They also contain glabridin compounds such as glycerin flavone, glabrene, glabryl, formononetin, and isoliquiritigenin
[14][68]. Many of these compounds have several known benefits and have been used as neuroprotective, antidepressive, oestrogenic, sedative, antimicrobial, specifically antiviral, anticarcinogenic, immunoregulatory, hepatoprotective, and antioxidant properties
[7][40][62,94].
2. Antimicrobial Activity
Many studies revealed the antibacterial activity of the plant (various extracts and flavonoids) against several bacteria strains, including
Helicobacter pylori and methicillin-resistant
Staphylococcus aureus (MRSA)
[3][30][38][41][42][43][58,84,92,95,96,97]. Mass and Cock
[37][91] studied the antibacterial efficacy of Licorice and demonstrated that the acetate root extract exhibited strong antibacterial efficacy against
K. pneumonia and
A. baylyi, and Wu et al.
[38][92] found that the flavonoids Glabrol, Licochalcone A, C, and E are very effective against
Staphylococcus aureus. The activity of GA against MRSA was studied by Long et al.
[30][84] and they found that the acid inhibits MRSA survival and attenuates virulent gene expression. Later, Celik and Duran
[29][83] found that the same GA is very effective against
Helicobacter pylori. Zhou et al.
[44
| [ |
| 68 |
| ] |
| [ |
| 122 |
| ] |
| |
| Cytomegalovirus (CMV) |
[ | 69][123] |
| |
Coxasackievirus B3 (CVB3) |
[70][124] |
| |
Coxasackievirus A16 (CVA16) |
[2][57] |
| Glycyrrhizic acid |
H5N1 influenza virus |
[50][51][52][104,105,106] |
| |
Duck Hepatitis virus |
[54][108] |
| |
Herpes simplex virus–1 |
[53][107] |
| |
|
[71][125] |
| Water extract |
HSV |
[55][109] |
| 18β-glycyrrhetinic acid |
Rotavirus |
[72][126] |
In Vivo Studies
The effect of GL on the influenza virus was conducted in a mouse model study more than two decades ago. The mice were treated with 10 mg of GL/kg body weight intraperitoneally (IP) one day before exposure to the virus (lethal dose able to kill around 50% of the animals). The result was successful, with all the GL-treated animals surviving the experimental period of 21 days, whereas, for the control animals, the mean survival time was only 10.5 days
[73][127]. The efficacy study of GL in murine herpes encephalitis revealed that the IP administration of GL increased the survival rate of animals by 2.5 times, and the viral replication in the brain was found to reduce more than 45%
[74][128]. A study with Coxsackievirus B3 (CVB3) revealed that GL is a factor in improving the state of Coxsackievirus B3 (CVB3)-induced myocarditis
[70][124].
Human Studies
During the SARS-CoV epidemic in China (2002–2003), Lu et al.
[75][129] conducted a clinical trial with GL against SARS-CoV on the virus confirmed patients. Among the 73 patients, 37 were treated with GL. After the complete treatment regimen, the major symptoms vanished quickly in the treated group compared to the placebo. Another trial was conducted on 60 SARS-CoV patients, with half belonging to the interventional group. The average period from peak severity of the lesions to 50% improvement was shorter in the interventional group treated with GL
[76][130].
3. Anti-Inflammatory Property
Inflammation plays a significant role in epidemic and pandemic diseases, and licorice is considered an alternative choice for the treatment
[7][77][62,131]. Inflammation is primarily a protective measure against microbial invasion, which includes action against the presence of toxins or allergens. However, in some cases, such as COVID-19, it may become uncontrollable and detrimental to the tissues and organs
[78][79][132,133]. It is the primary cause of many human diseases such as asthma, rheumatoid arthritis, and atherosclerosis
[80][134]. The inflammatory response occurs as a result of the production of pro-inflammatory cytokines such as IL-1, IL-6, IL-12, IL-18, interferon (INF)-γ, tumor necrosis factor (TNF), and granulocyte-macrophage colony-stimulating factor
[80][81][134,135]. The activity of the nuclear factor-kB (NF-kB) and transcription factors also play a significant role in inflammation by regulating the expression of various genes that encode the pro-inflammatory cytokines, adhesion molecules, chemokines, growth factors, and inducible enzymes such as cyclooxygenase-2 (COX-2)
[81][135].
Since ancient times, licorice has alleviated pain, relieving coughing, eliminated phlegm, and treated respiratory, liver, and gastric diseases
[82][136]. Like its antimicrobial activity, the anti-inflammatory activity (microbial induced inflammation) of licorice and its mechanism has been studied by many researchers
[4][7][83][84][85][59,62,137,138,139]. It has been found that the action is similar to those of glucocorticoids and mineralocorticoids and is mainly due to the presence of GL
[85][86][87][139,140,141] and GA in Licorice
[4][87][59,141]. Glabridin, liquiritin, liquiritigenin, and licochalcone, including 13 flavonoids, are present in licorice, in addition to GL and GA, and all these compounds have shown significant anti-inflammatory activity
[7][32][88][62,86,142]. Therefore, these compounds are used against liver and renal complications
[89][143]. The following are some of the studies that highlight GL and GA’s influence in treating various inflammatory diseases.
3.1. In Vitro Studies
There are several in vitro studies conducted to investigate the anti-inflammatory property of licorice, specifically the effects of GL and GA. Wang et al.
[90][144] investigated the same effects in lipopolysaccharide (LPS) stimulated macrophage model on RAW264.7 cells by treating with 25–75 µM GA or 18βGA and found that both are potential agents for the treatment of inflammatory-mediated diseases. They realized that both compounds inhibited the NF-kB activation and the activities of phosphoinositide-3-kinase (P13K) and reduced the production of LPS-induced tumor necrosis factor-ἁ (TNF-ἁ), IL-6, and IL-1β in a dose-dependent manner
[90][144]. Similarly, Bai et al.
[91][145] explored the anti-inflammatory effect of licorice residues and reported that a compound in the residue (compound 18) displayed the highest anti-inflammatory effect (No inhibitory effect) in the RAW264.7 cells. Further studies revealed that the anti-inflammatory effect happened through the downregulation of the pro-inflammatory cytokines (IL-1β, IL-6, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2))
[91][145]. Apart from GL and GA, one licorice extract named licoflavanone also showed strong anti-inflammatory activity in LPS-stimulated RAW 264.7 murine macrophages
[92][146].
3.2. Animal Studies
Aly et al.
[4][59] studied the anti-inflammatory activity of licorice using the carrageenan-induced edema model in male albino rats at the Al-Isra University in Jordan. They found that aqueous licorice extract and GA in licorice demonstrated significant anti-inflammatory activity similar to diclofenac sodium (DS). Similarly, in a mouse model study, Xiao et al.
[93][147] investigated the influence of GA in
Propionibacterium acnes-induced acute inflammatory liver injury. They found that GA exhibits anti-inflammatory effects through the inhibition of pro-inflammatory cytokines (such as IFN-γ and TNF-ἁ), P. acnes-induced NF-kB activation, and chemokine expression (MIP-1ἁ). Another investigation on the anti-inflammatory effects of GL found that it significantly inhibited the LPS-induced inflammatory response in a mouse by inhibiting the TLr4 signaling pathway
[94][148].
The anti-inflammatory activity of GA and hydroxypropyl γcyclodextrine was investigated against small intestine injury on indomethacin-treated mice. A significantly high plasma concentration of GA was detected after the oral administration of the compound
[95][149]. It was also found that 18β-glycyrrhetinic acid-hydroxypropyl-γcyclodextrin compound reduced the mRNA expression of the IL-6, IL-1β, including TNF-ἁ and thus showed a potential therapeutic value against indomethacin-induced small intestine injury
[95][149]. The ethanol extract of roasted licorice was also reduced in the TNF-ἁ and IL-6 and increased IL-10 in LPS treated mice, which facilitated the survival rate
[96][150]. Apart from this, many other studies also showed the anti-inflammatory property of licorice on live animals
[97][151], and the details are summarized in
Table 3.
It was reported that IP administration of GL suppressed the lung inflammation caused by the infection of
Streptococcus aureus in a mouse model study
[98][152]. Further to this, it has been mentioned that GL has a protective effect against TLR4 activator LPS-induced acute respiratory distress syndrome (ARDS) in mice
[99][153]. Similarly, in a mouse model experiment, it is observed that GL reduced the mortality of influenza-infected mice by interferon γ and T cell activation
[73][127]. Menegazzi et al.
[100][154] injected carrageenan (a well-known acute model inflammation widely used for inflammatory research) into the pleural cavity of mice to investigate GL’s influence in reducing inflammation, and they observed that the injection resulted in inflammation with fluid accumulation in the pleural cavity, mainly as a result of the production and accumulation of TNF-ἁ and IL-1β. The researchers noted that these inflammation events occurred as a result of the activation of NF-kB, including the activation of signal transducer and activator transcription-3 (STAT-3) in the lung
[100][154]. However, surprisingly GL inhibits these activation results in the reduction of inflammatory response.
Table 3. The anti-inflammatory property of licorice extracts in various studies.
| Compound |
Tissue/Disease |
Concentration |
Method |
Inhibition Rate |
Reference(s) |
| In vitro studies |
| 8β-GL |
LPS (1 μg mL−1)-induced l
Murine cell (RAW 264.7) |
75 μM |
ELISA |
51%-NO, 51%-IL-1β, 49%-PGE2 & 42%-IL-6 |
[90][144] |
| 18β-GL |
LPS (1 μg mL−1)-induced murine Cell (RAW 264.7 macrophages) |
0.5 or 1 mg mL−1 |
ELISA |
Supress PGE2, PGI2,TXB2 & LTB4 |
[101][155] |
| 18β-GL |
Leishmania donovani infected Macrophages-BALB/c mice (age: 4–6 weeks) |
50 mg mL−1 |
ELISA |
90.94%-parasite load |
[102][156] |
| 18β-GA |
LPS (1 μg mL−1)-induced
murine cell (RAW 264.7 cell) |
75 μM |
ELISA |
34%-NO |
[90][144] |
| 18β-GA |
|
75 μM |
ELISA |
58%-PEG2, 42%-1L-1β, 35%-IL-6, 34%-TNF-ἁ |
[90][144] |
| LID |
LPS (0.1 μg mL−1)-induced U937 Cell line (human monoblastic leukaemia cell line) |
0.1, 0.5, 1 μg mL−1 |
|
Decreased the secretion of IL-6, MMP-7, MMP-8, & MMP-9 |
[103][157] |
| DGC |
Glutamte (5 nM)-induced
HT22 cells |
2 μM |
2,7-DCF assay |
Dose-dependent inhibition of ROS assay & WB production |
[104][158] |
| LIA |
LPS (0.1 μg mL−1)-induced
U937 cell line (human monoblastic leukaemia cell line) |
0.1, 0.5, 1 μg mL−1 |
|
Decreased the secretions of CCL5 @ (1 μg mL−1), MMP-7 @ (0.5, 1 μg mL−1) MMP-8 @ (0.5, 0.1, 1 μg mL−1) |
[103][157] |
| LCA |
LPS (μg mL−1) induced murine cells (RAW 264.7) |
3 &10 μM |
DCFH-DA |
>80% PGE2 inhibition @ 10 μM fluorometric >50% NO inhibition at |
[97][151] |
| 18β-GL |
LPS (μg mL−1) induced |
75 μm |
ELISA |
51% reduction in NO |
[90][144] |
| |
Murine cells (RAW 264.7 cells) |
|
|
51% reduction in IL-1β
49% reduction in PGE2
46% reduction in TNF-ἁ
42% reduction in IL-6 |
|
| 18β-GA |
|
75 μm |
ELISA |
58% reduction in PEG2 |
[105][159] |
| Glabridin & isoliquiritigenin |
|
20-40 μg mL−1 |
Cell culture & cell viability assay |
anti-inflammatory activity is due to the individual or synergistic effects |
|
| In vivo studies |
| Compounds |
Inflammation Details |
Models |
Treatment |
Result(s) |
Reference |
| 18ἁ-GL |
20% paraquat poisoning solution @ 15 mg kg−1 |
Sprague Dawley rats-male 30 Ns (180–200 g) |
injection-IP 30 mg kg−1 |
Significant decrease in intercellular adhesion molecules (ICAM-1) and matrix metalloproteinase-9 (MMP-9) |
[93][147] |
| 18β-GL |
LPS (1 mg kg−1)-Intratracheal installation |
BALB/C mice (male 20–25 gm) |
injection-IP 10, 25 & 50 mg kg−1 |
Noted decrease in NO and MPO activity |
[106][160] |
| LCA |
Topical inflammation induced instantly at the posterior surface of the ear (using xylene 0.05 mL) |
Kunming mice (20–25 gm) & Wistar rats (150–200 gm) |
50 mg kg−1 |
Decrease in ear oedema rate by 30.3% |
[107][161] |
| Human Studies |
| GL |
Hepatitis B virus induced inflammation |
Humans |
oral and IV (60 mL daily) for a week) |
Effective in normalizing serum (for 7 days, later 3 days transaminases) |
[61][115] |
| GL |
Hepatitis C virus induced inflammation |
Humans |
40 mL transaminases |
Found effective in normalizing serum |
[62][116] |
| GL |
Hepatitis virus induced Inflammation |
Humans |
40 mg of GL (IV) |
Suppressed ALT |
[26][80] |
3.3. Human Studies
The influence of licorice extracts, GL and GA, on humans was studied by many researchers, who found that both effectively inhibit the viral replication and inflammatory response
[27][60][108][81,114,162]. Miyake et al.
[26][80] conducted a study that administered 40 mg of GL by injection to patients with chronic viral hepatitis and evaluated dose-response levels, including the frequency of administration. They found that GL effectively suppressed the alanine aminotransferase (ALT) in patients
[26][80]. Zhang and Wang
[28][82] also mentioned the efficacy of GC in lowering the ALT levels in chronic hepatitis B patients. In the LPS model of inflammation, GL can reduce TLR4 expression in the lung and the heart by significantly reducing the cytokine release
[99][109][153,163].
4. Effect on Autophagy
Autophagy is a cellular mechanism that cells use to adapt to stress conditions, including the internal invasion of pathogens. The process helps to clear out the pathogens, thereby reducing pathogen replication and the subsequent inflammatory consequences. However, some viruses like SARS-CoV-2 and HSV-1 can inhibit autophagy mechanisms to suit their replication conditions. This mechanism is usually linked to the inhibition of Beclin-1, which is considered one of the first proteins that a cell produces in the autophagic process
[110][111][185,186]. In addition to its antiviral and anti-inflammatory properties, it is found that the GL can induce the autophagy mechanism in the cells by increasing the concentration of Beclin-1
[53][107]. After 24 h of treatment with GL, it was observed that the Beclin-1 production significantly increased (
p < 0.01) (by two-fold to three-fold) in comparison with rapamycin treatment (
Figure 1 in
[53][107]). This is an important requirement in SARS-CoV-2 infected cells, as the SARS-CoV-2 virus inhibits Beclin-1 levels by inducing the production of SKP2 proteins
[112][113][187,188]. When GL was added to the cell 24 h before adding the herpes simplex virus (HSV1), Becklin production increased, and the cell demonstrated higher antiviral effects. The pre-treatment (2mM) increased the Beclin-1 production around five-fold compared to that induced at time zero. When GL was added to the HeLa cell together with HSV1, a strong antiviral activity was exhibited, whereas by comparison, the more traditional rapamycin treatment did not show any activity
[53][107]. This finding highlights GL’s ability to act as a prophylactic against HSV1, and it is suggested that it may also work against other viruses, including SARS-CoV-2.