Healthcare-associated infections (HAIs) are caused by nosocomial pathogens. HAIs have an immense impact not only on developing countries but also on highly developed parts of world. They are predominantly device-associated infections that are caused by the planktonic form of microorganisms as well as those organized in biofilms. This review elucidates the impact of HAIs, focusing on device-associated infections such as central line-associated bloodstream infection including catheter infection, catheter-associated urinary tract infection, ventilator-associated pneumonia, and surgical site infections associated with their peculiar microorganisms along with conventional and novel therapies.
- nosocomial infection
- medical devices
- catheter
- ventilation
- microorganisms
- biofilm
- treatment
1. Definition:
2. Introduction:
HAIs typically occur from 48 h to 30 days post-treatment [4][5]. In Europe, the European Centre for Disease Prevention and Control (ECDC) created in 2005 “The Healthcare-Associated Infections Surveillance Network”, and in the USA, the World Health Organization (WHO)’s section on prevention of hospital-acquired infections published their practical guide (second edition) in 2012. The WHO have stated that HAIs are a global phenomenon responsible for a high amount of morbidity and mortality all over the world [6]; they are a major problem not only in developing countries but also in the highly developed countries in Europe [4][7][8]. The ECDC reported that around 3.2 million patients acquired HAIs in European Union (EU) countries, and among them, 37,000 mortalities have been registered every year [9]. These infections also cause economic losses in the hospital sector, which have been increasing every year. A survey performed by Duszynska et al. (2020) revealed that one in five patients are diagnosed with a device-associated HAI, which leads to an additional financial burden of over 10,000 euros per patient [10]. Hopmans et al. (2020) conducted a biannual point-prevalence surveillance on HAIs from 2007 to 2016 and identified a decrease in the incidence of HAIs in hospitals in the Netherlands, specifically in surgical site infections (SSIs) and urinary tract infections (UTIs). This survey showed a reduction in the mean length of hospital stay by 3 days, but the usage of antibiotics increased from 31% to 36%. In this way, they demonstrated the importance of surveys to know the impact of ongoing treatment procedures to help and modify those procedures to provide a high standard of healthcare [11].
3. Content:
3.1 Medical devices associated infections:
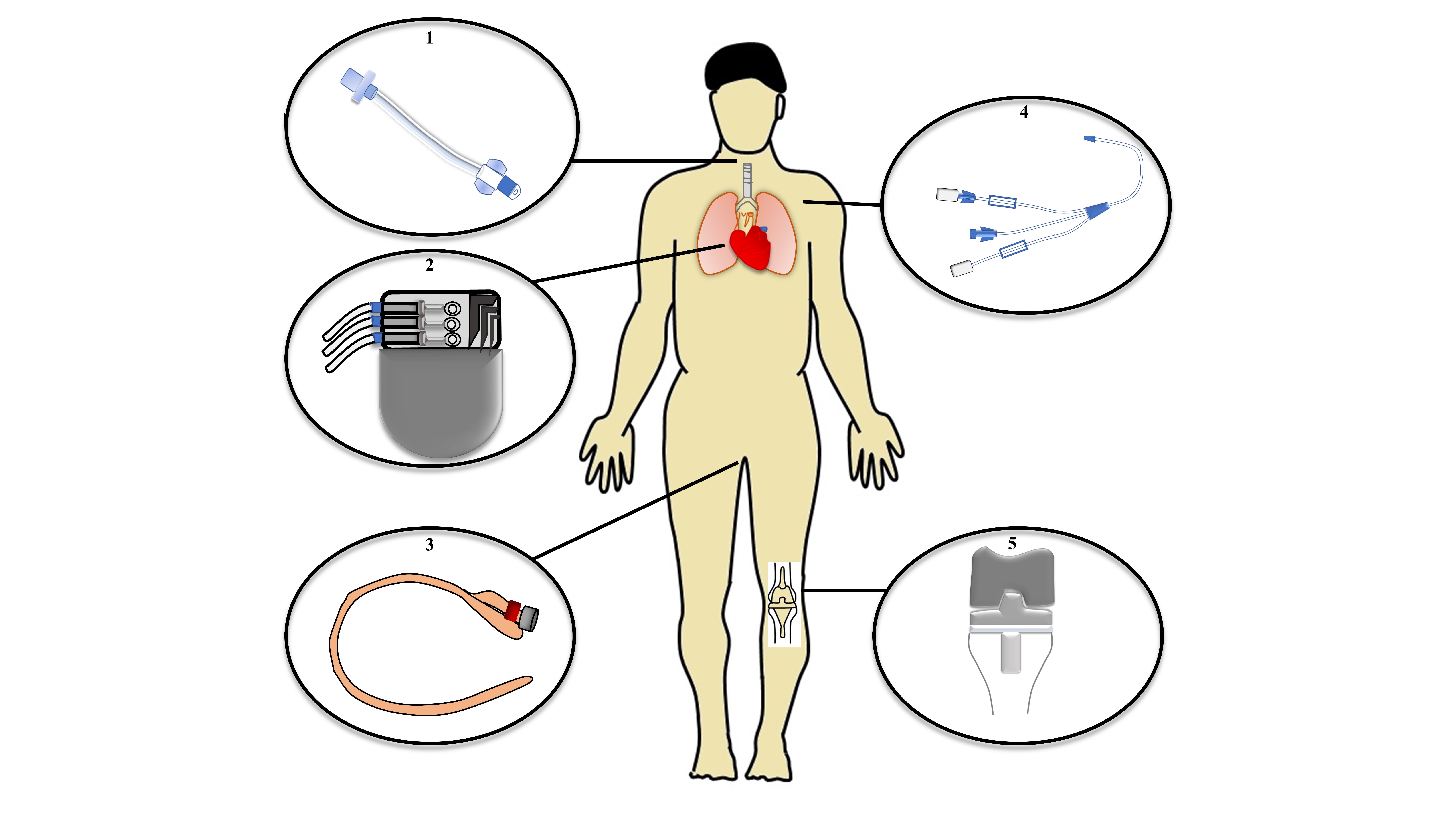
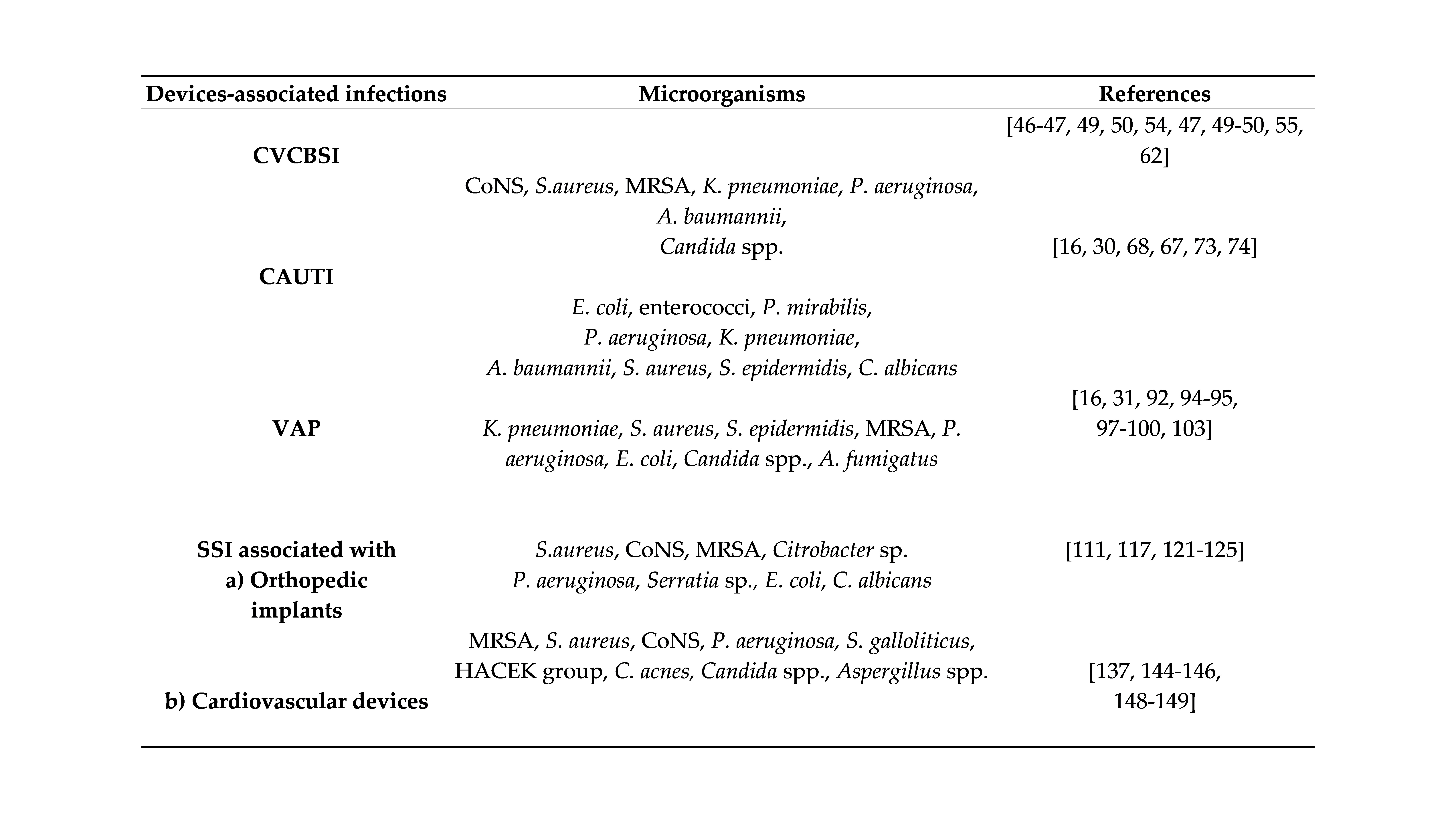
4. Conclusions
5. Funding
References
- Kollef, M.H.; Torres, A.; Shorr, A.F.; Martin-Loeches, I.; Micek, S.T. Nosocomial Infection. Crit. Care Med. 2021, 49, 169–187.
- Monegro, A.F.; Muppidi, V.; Regunath, H. Hospital Acquired Infections; StatPearls Publishing: Treasure Island, FL, USA; Las Vegas, NV, USA, 2021.
- European Centre for Disease Prevention and Control. Surveillance of Healthcare-Associated Infections and Prevention Indicators in European Intensive Care Units Year: Hai-Net Icu Protocol; Version 2.2; ECDC: Stockholm, Sweden, 2017; Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-healthcare-associated-infections-and-prevention-indicators-european (accessed on 23 September 2021).
- Walter, J.; Haller, S.; Quinten, C.; Kärki, T.; Zacher, B.; Eckmanns, T.; Abu Sin, M.; Plachouras, D.; Kinross, P.; Suetens, C. Healthcare-Associated Pneumonia in Acute Care Hospitals in European Union/European Economic Area Countries: An Analysis of Data from a Point Prevalence Survey, 2011 to 2012. Eurosurveillance 2012, 32, 1700843.
- Suetens, C.; Latour, K.; Kärki, T.; Ricchizzi, E.; Kinross, P.; Moro, M.L.; Jans, B.; Hopkins, S.; Hansen, S.; Lyytikäinen, O.; et al. Prevalence of Healthcare-Associated Infections, Estimated Incidence and Composite Antimicrobial Resistance Index in Acute Care Hospitals and Long-Term Care Facilities: Results from Two European Point Prevalence Surveys, 2016 to 2017. Eurosurveillance 2018, 23, 1800516.
- World Health Organization. Prevention of Hospital-Acquired Infections: A Practical Guide, 2nd ed.; World Health Organization: Geneva, Switzerland, 2002; Available online: https://www.who.int/csr/resources/publications/drugresist/WHO_CDS_CSR_EPH_2002_12/en/ (accessed on 30 March 2020).
- Badia, J.M.; Casey, A.L.; Petrosillo, N.; Hudson, P.M.; Mitchell, S.A.; Crosby, C. Impact of Surgical Site Infection on Healthcare Costs and Patient Outcomes: A Systematic Review in Six European Countries. J. Hosp. Infect. 2017, 96, 1–15.
- Zingg, W.; Hopkins, S.; Gayet-Ageron, A.; Holmes, A.; Sharland, M.; Suetens, C.; Almeida, M.; Asembergiene, J.; Borg, M.A.; Budimir, A.; et al. Health-Care-Associated Infections in Neonates, Children, and Adolescents: An Analysis of Paediatric Data from the European Centre for Disease Prevention and Control Point-Prevalence Survey. Lancet Infect. Dis. 2017, 17, 381–389.
- European Centre for Disease Prevention and Control. Economic Evaluations of Interventions to Prevent Healthcare-Associated Infections: Literature Review; Publications Office: Stockholm, Sweden, 2017.
- Duszynska, W.; Rosenthal, V.D.; Szczesny, A.; Zajaczkowska, K.; Fulek, M.; Tomaszewski, J. Device Associated –Health Care Associated Infections Monitoring, Prevention and Cost Assessment at Intensive Care Unit of University Hospital in Poland (2015–2017). BMC Infect. Dis. 2020, 20, 761.
- Hopmans, T.E.M.; Smid, E.A.; Wille, J.C.; Kooi, T.I.I.; van der Koek, M.B.G.; Vos, M.C.; Geerlings, S.E.; Greeff, S.C. De Trends in Prevalence of Healthcare-Associated Infections and Antimicrobial Use in Hospitals in the Netherlands: 10 Years of National Point-Prevalence Surveys.2020. J. Hosp. Infect. 2020, 104, 181–187.
- Vandecandelaere, I.; Matthijs, N.; van Nieuwerburgh, F.; Deforce, D.; Vosters, P.; de Bus, L.; Nelis, H.J.; Depuydt, P.; Coenye, T. Assessment of Microbial Diversity in Biofilms Recovered from Endotracheal Tubes Using Culture Dependent and Independent Approaches. PLoS ONE 2012, 7, e38401.
- Holá, V.; Ruzicka, F.; Horka, M. Microbial Diversity in Biofilm Infections of the Urinary Tract with the Use of Sonication Techniques. FEMS Immunol. Med. Microbiol. 2010, 59, 525–528.
- Boev, C.; Kiss, E. Hospital-Acquired Infections: Current Trends and Prevention. Crit. Care Nurs. Clin. N. Am. 2017, 29, 51–65.
- Gentili, A.; Di Pumpo, M.; La Milia, D.I.; Vallone, D.; Vangi, G.; Corbo, M.I.; Berloco, F.; Cambieri, A.; Damiani, G.; Ricciardi, W.; et al. A Six-Year Point Prevalence Survey of Healthcare-Associated Infections in an Italian Teaching Acute Care Hospital. Int. J. Environ. Res. Public Health 2020, 17, 7724.
- Böll, B.; Schalk, E.; Buchheidt, D.; Hasenkamp, J.; Kiehl, M.; Kiderlen, T.R.; Kochanek, M.; Koldehoff, M.; Kostrewa, P.; Claßen, A.Y.; et al. Central Venous Catheter-Related Infections in Hematology and Oncology: 2020 Updated Guidelines on Diagnosis, Management, and Prevention by the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 2021, 100, 239–259.
- Wendel, D.; Mezoff, E.A.; Raghu, V.K.; Kinberg, S.; Soden, J.; Avitzur, Y.; Rudolph, J.A.; Gniadek, M.; Cohran, V.C.; Venick, R.S.; et al. Management of Central Venous Access in Children With Intestinal Failure: A Position Paper From the NASPGHAN Intestinal Rehabilitation Special Interest Group. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 474–486.
- Menegueti, M.G.; Ciol, M.A.; Bellissimo-Rodrigues, F.; Auxiliadora-Martins, M.; Gaspar, G.G.; Canini, S.R.M.; da Silva Canini, S.R.M.; Basile-Filho, A.; Laus, A.M. Long-Term Prevention of Catheter-Associated Urinary Tract Infections among Critically Ill Patients through the Implementation of an Educational Program and a Daily Checklist for Maintenance of Indwelling Urinary Catheters: A Quasi-Experimental Study. Medicine 2019, 98, e14417.
- Gad, M.H.; AbdelAziz, H.H. Catheter-Associated Urinary Tract Infections in the Adult Patient Group: A Qualitative Systematic Review on the Adopted Preventative and Interventional Protocols From the Literature. Cureus 2021, 13, e16284.
- Letica-Kriegel, A.S.; Salmasian, H.; Vawdrey, D.K.; Youngerman, B.E.; Green, R.A.; Furuya, E.Y.; Calfee, D.P.; Perotte, R. Identifying the Risk Factors for Catheter-Associated Urinary Tract Infections: A Large Cross-Sectional Study of Six Hospitals. BMJ Open 2019, 9, e022137.
- Vincitorio, D.; Barbadoro, P.; Pennacchietti, L.; Pellegrini, I.; David, S.; Ponzio, E.; Prospero, E. Risk Factors for Catheter-Associated Urinary Tract Infection in Italian Elderly. Am. J. Infect. Control 2014, 42, 898–901.
- Medina-Polo, J.; Gil-Moradillo, J.; González-Díaz, A.; Abad-López, P.; Santos-Pérez de la Blanca, R.; Hernández-Arroyo, M.; Peña-Vallejo, H.; Téigell-Tobar, J.; Calzas-Montalvo, C.; Caro-González, P.; et al. Observational Study over 8-Year Period Evaluating Microbiological Characteristics and Risk Factor for Isolation of Multidrug-Resistant Organisms (MDRO) in Patients with Healthcare-Associated Infections (HAIs) Hospitalized in a Urology Ward. GMS Infect. Dis. 2021, 9, Doc04.
- Li, Y.-C.; Lin, H.-L.; Liao, F.-C.; Wang, S.-S.; Chang, H.-C.; Hsu, H.-F.; Chen, S.-H.; Wan, G.-H. Potential Risk for Bacterial Contamination in Conventional Reused Ventilator Systems and Disposable Closed Ventilator-Suction Systems. PLoS ONE 2018, 13, e0194246.
- de Souza Kock, K.; Maurici, R. Respiratory Mechanics, Ventilator-Associated Pneumonia and Outcomes in Intensive Care Unit. World J. Crit. Care Med. 2018, 7, 24–30.
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-Associated Infections, Medical Devices and Biofilms: Risk, Tolerance and Control. J. Med. Microbiol. 2015, 64, 323–334.
- Ling, M.L.; Apisarnthanarak, A.; Jaggi, N.; Harrington, G.; Morikane, K.; Thu, L.T.A.; Ching, P.; Villanueva, V.; Zong, Z.; Jeong, J.S.; et al. APSIC Guide for Prevention of Central Line Associated Bloodstream Infections (CLABSI). Antimicrob. Resist. Infect. Control 2016, 5, 16.
- Pinto, H.; Simões, M.; Borges, A. Prevalence and Impact of Biofilms on Bloodstream and Urinary Tract Infections: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 825.
- Svensson Malchau, K.; Tillander, J.; Zaborowska, M.; Hoffman, M.; Lasa, I.; Thomsen, P.; Malchau, H.; Rolfson, O.; Trobos, M. Biofilm Properties in Relation to Treatment Outcome in Patients with First-Time Periprosthetic Hip or Knee Joint Infection. J. Orthop. Transl. 2021, 30, 31–40.
