Fungal keratitis is a serious clinical infection on the cornea caused by fungi and is one of the leading causes of blindness in Asian countries. The treatment options are currently limited to a few antifungal agents. With the increasing incidence of drug-resistant infections, many patients fail to respond to antibiotics. Riboflavin-mediated corneal crosslinking (similar to photodynamic therapy (PDT)) for corneal ectasia was approved in the US in the early 2000s. Current evidence suggests that PDT could have the potential to inhibit fungal biofilm formation and overcome drug resistance by using riboflavin and rose bengal as photosensitizers.
1. Introduction
According to the World Health Organization (WHO), around 6 million people globally are affected by cornea-related blindness
[1]. Corneal opacity is estimated to be responsible for 1.5–2.0 million new cases of monocular blindness each year
[1], with etiologies including infection, trauma, inflammation, degeneration, and nutritional deficiency
[1]. Among the etiologies, infectious keratitis (IK) stands at the top with an estimated incidence of 2.5–799 per 100,000 population-year
[2]. IK can be caused by pathogens, such as bacteria, fungi, virus, parasites, and polymicrobial infections, which may vary depending on different geographic locations and seasons
[3].
Bacterial infections make up 79–100% of IK, depending on the country and study period
[1]. Fungal keratitis, on the other hand, is more prevalent in Asian countries
[2][3][2,3]. It is a serious corneal fungal infection, commonly caused by
Candida,
Fusarium, and
Aspergillus, that often results in blindness and eye loss, especially in developing countries
[4]. The global minimal annual incidence is estimated at 1.05 million cases, with the highest rates in Asia and Africa. Even with the advancement of biotechnology, there are few antifungal agents available, including natamycin, amphotericin B, fluconazole, and voriconazole
[1]. The situation is complicated by the rapid emergence of drug-resistant fungal keratitis globally
[5][6][5,6], to the extent that some patients require a full thickness corneal transplantation (penetrating keratoplasty)
[7] as treatment. In their 1991 study, Kirkness et al. suggested early intervention with corneal transplantation regarding the management of advanced microbial keratitis
[8]. The overall success rate is around 80–90%
[7][9][7,9], however graft failure and the recurrence of infection could occur in an active infected eye after operation
[10]. Furthermore, the acquired and innate antifungal drug resistance has drastically increased over the past three decades
[2]. Moreover, the clinical response to fungal infection does not always correlate with in vitro drug sensitivity testing
[11][12][11,12]. Hence, new and novel therapies are crucially required to treat and prevent drug-resistant fungal infections.
Photodynamic therapy (PDT) comprises the activation of a specific photosensitizer (PS) with an absorption peak light wavelength of the PS in the presence of oxygen molecules in the tissue and has been widely used to kill cancer cells for three decades
[13]. The application of PDT against microorganisms can be dated back to the 1900s when Rabb showed photodynamic effects after exposing
Paramecium caudatum to acridine or eosin dyes and illuminated them with sunlight
[14]. Even though antimicrobial PDT (aPDT) has shown great potential in treating drug-resistant infectious diseases in vitro and in animal studies, only a few clinical trials are currently ongoing
[14][15][14,15]. Yet, aPDT has several advantages: (1) It is a local treatment with extremely rare systemic side effects; (2) The antimicrobial effects are medicated by the generation of singlet oxygen and reactive oxygen species (ROS) during irradiation, which damage multiple organelles in a cell, thus PDT resistance has not yet been reported; (3) It functions well both in targeting against planktonic and in biofilm microorganisms
[14][15][14,15]; (4) Bacteria survive after PDT reduced resistance to antibiotics
[16], and some PSs bind more rapidly and selectively to microbials than human cells
[14]. So, the killing of the microbials is highly selective in aPDT.
In the field of ophthalmology, PDT was introduced to treat choroidal neovascularization in the 1990s
[17]. Before then, the role of PDT in eliminating ocular infection had been rarely studied. Riboflavin-mediated corneal crosslinking (CXL), which is a form of PDT, utilizes riboflavin eye drops as a PS and activates with ultraviolet-A (UVA) to increase the stiffness of the cornea
[18]. After its introduction in 2003 by Theo Seiler
[18], the application of CXL was extended to IK
[19]. Recently, aPDT that utilizes rose bengal as a PS and activation with green light has shown a 72% success rate in IK patients
[20][21][20,21].
2. Fungal Keratitis
Fungal keratitis was first described by Leber in 1879. It is a serious corneal infection with poor visual prognosis
[1][22][23][24][1,22,23,24], causing a significant socioeconomic burden, especially in developing countries because it commonly affects young male outdoor agricultural workers
[25]. The incidence of fungal keratitis has increased over the past three decades due to the frequent use of topical corticosteroids and antibiotics in IK treatment. The estimated minimum annual incidence is around 1 million worldwide with the highest rates in Asia and Africa, and the loss of around 84,143–115,697 eyes
[4]. The proportions of fungal keratitis in IK vary from less than 10% in temperate regions to more than 45% in tropical and subtropical regions
[4]. The most common pathogens causing fungal keratitis are filamentous fungi (
Fusarium,
Aspergillus) and yeasts (
Candida albicans and other
Candida species)
[1]. Fungi enter corneal stroma through the epithelial defect or extend from the posterior segment through the descent membrane (fungal endophthalmitis). Another entry pathway is through corneoscleral trabeculae into the corneal channel network, since trauma to the corneal epithelium by a contaminated sharp object is very common in farmers in developing countries. In addition to trauma, risk factors for fungal keratitis include pre-existing ocular disorders, systemic disorders, wearing of contact lenses, topical steroid use, and recent ocular surgery
[9].
The treatment of fungal keratitis remains challenging because of the difficulty in early diagnosis, limited choices in anti-fungal agents, the emergence of antifungal drug tolerance and resistance
[6], and the formation of biofilm, which will be further elaborated in the following section. The mainstay medical treatment is topical anti-fungal agents, e.g., polyenes (amphotericin B, natamycin), triazoles (fluconazole, voriconazole, posaconazole), echinocandins (caspofungin, micafungin), and pyrimidine analogue (flucytosine) with or without systemic antifungal agents
[22][24][22,24]. Fusarium keratitis is difficult to treat because the
Fusarium spp. are intrinsically resistant to most antifungals, including echinocandins
[26][27][26,27]. Since the approval of natamycin in the 1960s by the US Food and Drug Administration, no new topical antifungal eye drops have been approved and natamycin is currently considered the most effective medication against
Fusarium [24].
Among the available antifungal agents, voriconazole has demonstrated the best ocular penetration and broadest coverage of fungal species in vitro
[23]. To overcome the disadvantage of poor corneal penetration of antifungal agents, intrastromal or intracameral drug injections have also been proposed
[28]. Even with the advancement of new drugs and a new methodology, 40–60% of fungal keratitis cases are refractory to medical therapy and require surgical intervention
[9][22][9,22], including multiple keratectomies or penetrating keratoplasty. For patients receiving therapeutic keratoplasty performed in an active infection stage, the five-year survival rate was only 51% compared to 90% in cases with inactive infection
[8]. Moreover, long-term use of immunosuppressants can lead to repetitive
Candida keratitis, which may require multiple corneal transplantations
[29]. Overall, fungal keratitis is associated with poorer visual outcomes and remains a great challenge to ophthalmologists
[22][24][26][22,24,26].
3. Drug Resistance and Biofilm in Fungal Keratitis
The emergence of drug resistance in fungal infection poses a significant threat to public health globally
[5][6][30][5,6,30]. Cases of multidrug resistant
Fusarium keratitis
[12][30][31][32][12,30,31,32] or azole-resistant
Candida keratitis
[33] are rare but can be very challenging once they occur. In cases with multidrug-resistant fungal keratitis, the visual outcome is generally devastating despite intense conventional treatments, even requiring some patients to undergo enucleation to control the infection.
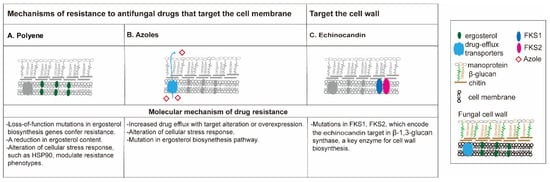
The mechanism of antifungal drug resistance (
Figure 1) is different among different classes of drugs
[34][35][34,35]. The polyenes are the oldest class of antifungal drugs and include amphotericin B and nystatin. Polyene drugs target ergosterol, a fungal-specific sterol synthesized in the plasma membrane. The sequestration of ergosterol leads to the increase of membrane permeability, eventually causing cell death. Resistance to polyenes is rarely reported, and mainly related to decreased membrane ergosterol, an alteration of cellular stress response (mutations in
ERG3 gene) as reported in
Candida [34][36][34,36]. In some cases, treatment with an azole antifungal, which in turn reduces ergosterol, can confer polyene resistance
[36].
Figure 1. Mechanisms of antifungal agent resistance. Polyenes (A) and azoles (B) are membrane targeting antifungal drugs while echinocandins are cell wall-active agents. (A) Polyene resistance is often attributed to loss-of-function mutations in ergosterol biosynthetic genes which lead to depletion of ergosterol, the fungi-specific cell membrane sterol. Resistance mechanisms for Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus are outlined in dashes. (B) Azole resistance can result from the upregulation of two classes of efflux pumps that remove the drug from the cell; through the mutation or overexpression of ERG11, which minimizes the impact of the drug on the target; or alterations in ergosterol biosynthesis, such as the loss-of-function mutation of ERG3, which blocks the accumulation of a toxic sterol intermediate that is produced when ERG11 is inhibited. (C) Resistance to echinocandins can result from mutations in FKS1 that minimize the impact of the drug on the target.
Azole antifungal agents are some of the most widely used antifungal agents, and offer activity against many fungal pathogens without the serious nephrotoxic effects observed with amphotericin B
[37]. The azoles available in the clinic can be classified into two groups: the triazoles (fluconazole, itraconazole, voriconazole, posaconazole, and isavuconazole) and the imidazoles (ketoconazole). The azole antifungals are also membrane-targeted, primarily by inhibiting the cytochrome P450-dependent enzyme lanosterol 14-alpha-demethylase, a critical enzyme that converts lanosterol to ergosterol
[38]. Triazole resistance is mainly caused by the increased activity of efflux pumps that remove the drug from the cell due to the overexpression or mutations of
ERG11 and
CYP51 genes, and/or the alteration of cellular stress response genes (loss-of-function of
ERG3 gene)
[6][35][6,35].
Echinocandins constitute the first class of antifungals to target the fungal cell wall. This class of antifungals inhibits β-(1,3)-
d-glucan synthase, a critical enzyme for the synthesis of polysaccharide β-(1,3)-
d-glucan, a component of the cell wall of many fungi. Three semi-synthetic echinocandins, namely caspofungin, micafungin, and anidulafungin, have been developed for clinical use and are usually reserved for invasive fungal keratitis
[39]. Clinical experience with this antifungal class suggests that it is among the best tolerated and safest classes of antifungals available
[40]. The acquired resistance to echinocandins remains sporadic and varies by region but is possibly increasing, especially among invasive
C. glabrata infections with
FKS1 and
FKS2 mutations
[6].
A biofilm is defined as a structured microbial community attached to a surface and encased within a self-produced extracellular matrix
[41][42][41,42], which blocks the entry of the antifungal agents
[43]. Fungi isolated from keratitis are able to produce biofilm
[44], impairing the susceptibility of antifungal agents, and protecting the fungi from UV light
[44], thus enhancing fungal resistance
[6]. The ability of fungi to form biofilms is correlated to their ability to form disease in humans
[45], irrespective of the thickness of these biofilms
[46]. Only a few antimycotics, such as miconazole (azoles), echinocandins, and liposomal formulations of amphotericin B (polyenes), have shown effectiveness against fungal biofilms
[47][48][47,48]. Pérez-Laguna et al. reviewed the combination of aPDT and antimicrobial compounds to treat skin and mucosal infections in humans or animals
[49]. They concluded that aPDT has additive or synergistic effects both in planktonic suspensions and biofilms, which may relate to an increase in membrane permeability by the aPDT in fluconazole-resistant
C. albicans strains. Interestingly, combination therapies with natural products may enhance antifungal agents against biofilm. Lactoferricin B, a peptide of bovine lactoferrin exhibiting multiple biological functions, including antimicrobial, antiviral, antioxidant, and immunomodulatory activities, has been proposed to improve biofilm susceptibility to antifungals
[50]. Other compounds, including monoterpenes, sesquiterpenes, extracts from microalgae, and Cyanobacteria, also showed enhancement of antifungal agents in fungal biofilm inhibition
[51][52][51,52]. Their mechanisms are believed to relate to the induction of ROS by antifungal agents and targeting the fungal oxidative defense system
[47].
Table 1 summarizes the treatment outcome of IK caused by multidrug-resistant fungi with traditional treatments.
Table 1. Outcomes of case reports affected by multidrug resistant fungal keratitis.
|
| Ref. (Year) Citation |
|
| Pathogens |
|
| Initial VA |
|
| Antifungal Drugs |
|
| Surgery |
|
| Outcome |
|
|
| Sponsel (2002) [30] |
|
| F. solani |
|
| Not mentioned |
|
| AMB-intravenous, topical |
| KTC-topical |
| NAT-topical |
| POS-PO, topical |
|
| PK |
|
| VA: 6/30 |
|
|
| Guarro (2003) [53] |
|
| F. polyphialidicum |
|
| 1/200 |
|
| AMB-topical |
|
| Corneal transplantation |
|
| VA: 20/40 (improved) |
|
|
| Tu (2007) [54] |
|
| F. solani |
|
| HM |
|
| AMB-IVI, topical |
| FLC-PO |
| ITC-PO |
| NAT-topical |
| POS-PO |
| VRC-intravenous, IVI, PO |
|
| PK for 3 times |
|
| VA: CF (improved) |
|
|
| VA: HM (2 out of 6 cases), LP (2 out of 6 cases), 6/60 (2 out of 6 cases) (~20% cases improved; ~20% cases stable disease, ~60% cases worsened) |
|
| Fusarium sp. |
|
| Not mentioned |
|
|
| Vajpayee (2015) [79] |
|
| Aspergillus sp., Fusarium sp. |
| AMB-topical |
| FLC-PO |
| NAT-topical |
| VRC-PO, topical |
| POS-PO, topical |
|
| Retrospective study. |
| PK for 2 times |
|
| 20 |
| Resolution of inflammation |
|
|
|
| 0.1% RFB |
|
| UVA (365 nm) |
| 3.0 mW/cm 2 |
| 30 min |
|
| BCVA: 1.13 ± 0.55 (stable disease) |
|
| Fusarium sp. |
|
| Not mentioned |
|
| AMB-AC injection, topical |
| CYA-topical |
| FLC-PO |
| NAT-topical |
| POS-PO |
| VRC-IVI, PO, topical |
|
| PK, penetrating patch graft |
|
| Poor vision, awaiting repeat corneal transplantation |
|
|
| Proença-Pina (2010) [55] |
|
| F. solani |
|
| HM |
|
| AMB-AC irrigation, topical |
| VRC-PO, topical |
|
| PK |
|
| VA: 20/50 (improved) |
|
|
| Edelstein (2012) [56] |
|
| F. solani |
|
| HM |
|
| AMB-ICI, IVI, topical |
| FLC-PO |
| ITC-PO |
| NAT-topical |
| POS-PO |
| VRC-PO, topical |
|
| PK for 2 times, pars plana vitrectomies, enucleation |
|
| Enucleation |
|
|
| Antequera (2015) [31] |
|
| F. solani |
|
| - |
|
| AMB-intravenous |
| CAS-intravenous |
| VRC-intravenous, PO, topical |
|
| Enucleation |
|
| Enucleation |
|
|
| Sara (2016) [12] |
|
| F. solani |
|
| 6/12 |
|
| AMB-IVI |
| NAT-topical |
| VRC-IVI, PO, topical |
|
| PK, enucleation |
|
| Enucleation |
|
AC: Anterior chamber; AMB: Amphotericin B; CAS: Caspofungin; CF: Counting fingers; CYA: Cyclosporine A; FLC: Fluconazole; HM: Hand movement; ICI: Intracameral injection; ITC: Itraconazole; IVI: Intravitreal injection; KTC: Ketoconazole; NAT: Natamycin; PK: Penetrating keratoplasty; PO: oral; POS: Posaconazole; VA: Visual acuity; VRC: Voriconazole.
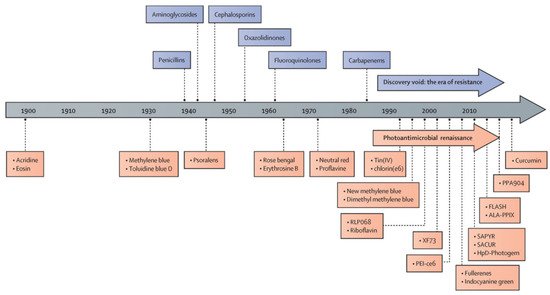
4. The History of Antimicrobial Photodynamic Therapy
Photodynamic therapy (PDT) has been used as a noninvasive treatment for the selective destruction of pathogenic organisms using a handful of non-toxic PSs since its earliest development (
Figure 2). After the discovery of penicillin in 1928, the golden era of antibiotics began in the 1940s and lasted until late 1960s with the development of different classes of antibiotics, including aminoglycosides, tetracyclines, chloramphenicols, sulfones, macrolides, glycopeptides, polymyxins, oxazolidinones, ansamycins, quinolones, azoles, and ethambutol
[15]. Similarly, the discovery of aPDT has been accelerated by the development of new classes of PSs since the 1900s, nowadays known as the era of drug resistance (
Figure 2). The PSs investigated during the era of aPDT renaissance include tetracationic Zn(II) phthalocyanine PS (RLP-068)
[57], methylene blue
[58], Tin(IV) porphyrins
[59], chlorine e6
[60], new formulations of methylene blue
[61], riboflavin
[62], exeporfinium chloride (XF73)
[63], fullerenes
[64], indocyanine green
[65], 2-((4-pyridinyl)methyl)-1H-phenalen-1-one chloride (SAPYR)
[66], curcumin derivative (SACUR-3)
[67], hematoporphyrin derivative-Photogem
[68], 5-aminolevulinic acid induced protoporphyrin IX (ALA-PpIX)
[69], C₂₈H₄₂BrN₃S, phenothiazin-5-ium, 3,7-bis(dibutylamino)-, bromide (PPA904)
[70], and curcumin
[71].
Figure 2. History of antimicrobial photodynamic therapy. RLP068: tetracationic Zn(II) phthalocyanine chloride; XF73: positively charged porphyrin; PEI-ce6: polyethyleneimine chlorin(e6) conjugate; SAPYR: perinapthenone derivative. SACUR: curcumin derivative; HpD-Photogem:haematoporphyrin derivative; FLASH: cationic riboflavin derivative; ALA-PPIX: 5-aminolevulinic acid-induced protoporphyrin IX; PPA90: tetrabutyl derivative of methylene blue. Reprinted from ref.
[14] in text with permission from the Publisher.
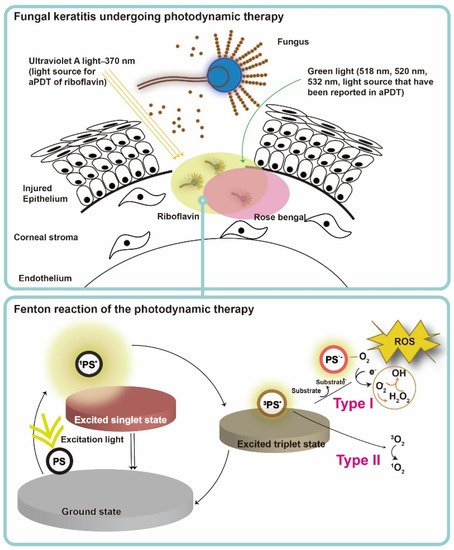
5. Mechanism of the Photodynamic Action in Fungal Infection
PS, light, and oxygen in tissue or in a cell are the three key elements of PDT, and none of them is toxic or cell/tissue damaging by itself. Upon excitation by light containing the absorption peaks of a PS (usually red or blue light, near-infrared light and even sunlight
[13]), the PS transforms from ground state to the short-lived singlet state, and then relaxed to the triplet state (PS*) (
Figure 3)
[67]. After achieving the triplet state of a PS, two kinds of reaction follow. In the type I reaction, the excited PS reacts through electron transfer with biomolecules, such as lipids, proteins, and amino acids, to yield the superoxide anion radical (O
2•−) and hydroxyl radical (•OH). O
2•− undergoes dismutation to form hydrogen peroxide (H
2O
2), the precursor of the highly reactive •OH. •OH is extremely chemically reactive to almost all biological molecules
[68]. In the type II reaction, the excited PS yields singlet oxygen (
1O
2) through a direct energy transfer to molecular oxygen. Like the hydroxyl radical,
1O
2 is highly reactive
[67]. These two types of reactions compete with each other, and the type II reaction is believed to be the principal mechanism of O
2-dependent PDT
[69]. In a microorganism, the photodynamic actions should take place where the PS deposited, as the half-life of singlet oxygen and ROS are only within microseconds and the diffusion distance is within micrometers
[70]. Therefore, PDT targets multiple organelles in a cell. No evidence of any PDT-resistant microorganisms has been reported so far. On the contrary, MRSA was reported to become more sensitive to antibiotics after ICG-mediated PDT, which was partly related to mecA complex gene deletion
[16].
Figure 3. Schematic illustration of antimicrobial photodynamic therapy mechanism for fungal keratitis. The ground-state photosensitizer (PS) absorbs photons and is excited to the first short-lived excited singlet state and either returns to the ground state or undergoes intersystem crossing to a long-lived triplet state. The triplet state PS exerts downstream function via a type I or type II photosensitization process. For type I reaction, charge is transferred from the excited PS to oxygen (O2), and therefore leading to the formation of hydrogen peroxide (H2O2), hydroxyl radical (HO·), and superoxide anion (O2−·). For type II reaction, the triplet PS undergoes energy exchange with triplet ground state oxygen, leading to the formation of singlet oxygen 1O2. Type I and type II reactions can occur at the same time during irradiation. Nevertheless, type II reaction is mainly involved in antimicrobial photodynamic action. The reaction depends most importantly on PS used and the concentration of O2 in aPDT.
6. Antimycotic Photodynamic Therapy
Most published studies of antimycotic PDT today focus on in vitro investigations
[72][73][72,73].
Table 2 summarizes the clinical applications of aPDT against fungal keratitis. PSs used in aPDT for IK include toluidine blue O (TBO), methylene blue (MB)
[74][75][74,75], rose bengal (RB)
[20], and riboflavin (RBF)
[76][77][76,77]. The following section focuses on PSs in antimycotic studies.
Table 2. Clinical reports of antimycotic photodynamic therapy for fungal keratitis.
|
| Ref. (Year) Citation |
|
| Pathogens |
|
| Study Type |
|
| Case Number |
|
| Photosensitizer |
|
| Light Source (Wavelength), |
| Irradiance, Irradiation Time or Radiant Exposure |
|
| Outcome |
|
|
| Iseli (2008) [19] |
|
| Acremonium sp. |
|
| Case reports |
|
| 1 |
|
| 0.1% RFB |
|
| UVA |
| 3.0 mW/cm 2 |
| 30 min |
|
| VA: CF after CXL, |
| 20/30 after DALK (8 months after CXL) (improved) |
|
|
| Fusarium sp. |
|
| 1 |
|
| 0.1% RFB |
|
| UVA |
| 3.0 mW/cm 2 |
| 30 min |
|
| Corneal infiltrate progressed after CXL |
| → PK |
|
|
| Uddaraju (2015) [78] |
|
| Aspergillus sp., Fusarium sp. |
|
| RCT |
|
| 6 |
|
| 0.1% RFB |
|
| UVA (370 nm) |
| 3.0 mW/cm 2 |
| 30 min |
|
|
| Kasetsuwan (2016) [80] |
|
| Fusarium sp., Aspergillus sp., Purpureocillium sp., Pythium sp. |
|
| RCT |
|
| 8 |
|
| 0.1% RFB |
|
| UVA (365 nm) |
| 3.0 mW/cm 2 |
| 30 min |
|
| Median size of stromal infiltration: |
| 30.2 mm 2→ 9.1 mm2 |
| Median size of epithelial defect: |
| 23.7 mm 2 → 1.42 mm 2 |
|
|
| Amescua (2017) [81] |
|
| Fusarium sp. |
|
| Case reports |
|
| 1 |
|
| 0.1% RB |
|
| Green light LED (518 nm) |
| 0.9 J/cm 2→ 1.8 J/cm2 |
|
| Clear cornea with fine endothelial function |
|
|
| Mikropoulos (2019) [82] |
|
| P. lilacinum |
|
| Case report |
|
| 1 |
|
| RFB |
|
| UVA |
| 9.0 mW/cm 2 |
| 30 min |
| (intraoperative) |
|
| VA: CF at 1 m (stable disease) |
|
|
| Naranjo (2019) [20] |
|
| Fusarium sp. |
|
| Consecutive case series. |
|
| 4 |
|
| 0.1% RB |
|
| Green light LED 6.0 mW/cm2 |
| 15 min |
|
| BCVA: 20/100, 20/800, HM, NLP (50% cases improved; 25% cases stable disease, 25% cases worsened) |
|
|
| Curvularia sp. |
|
| 1 |
|
| 0.2% RB |
|
| Green light LED 6.0 mW/cm2 |
| 15 min |
|
| BCVA: 20/50 (improved) |
|
|
| Prajna (2020) [83] |
|
| Aspergillus sp., Bipolaris sp., Colletotrichum sp., Curvularias sp., Exserohilum sp., Fusarium sp., Scedosporium sp. |
|
| RCT |
|
| 55 |
|
| 0.1% RB |
|
| UVA (365 nm) |
| 3.0 mW/cm 2 |
| 30 min |
|
| VA: 3.2 Snellen lines worse at 3 months than baseline VA (worsened in all cases) |
|
BCVA: Best-corrected visual acuity; CF: Counting fingers; CXL: Corneal crosslinking; DALK: Deep anterior lamellar keratoplasty; HM: Hand movement; LED: Light emitting diodes; LP: Light perception; NLP: No light perception; PK: Penetrating keratoplasty; RB: rose bengal; RCT: Randomized controlled trial; RFB: riboflavin; UVA: Ultraviolet A; VA: Visual acuity.