Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Yvaine Wei and Version 1 by Muhammad Umar.
Perfluoroalkyl and polyfluoroalkyl substances (PFASs) consist of a group of environmentally persistent, toxic and bio-accumulative organic compounds of industrial origin that are widely present in water and wastewater. Despite restricted use due to current regulations on their use, perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) remain the most commonly detected long-chain PFAS.
- PFAS
- UV
- VUV
- oxidation
- water
1. Introduction
Perfluoroalkyl and polyfluoroalkyl substances (PFASs) (C5–C18) are widely used in different industrial applications (clothing, paper packing, non-stick cookware, food packaging, pesticide formulations, waterproof fabrics, fume suppressants, photographic films, masking tape, firefighting foams) due to their unique properties [1,2][1][2]. These special properties of PFASs are associated with characteristics such as: (1) the hydrogen atoms on the alkyl chain are replaced by fluorine atoms [3], and (2) the presence of both long hydrophobic perfluorinated (CnH2n+1) carbon chain and hydrophilic functional group (-SO−3SO3−, -COO−), i.e., in PFOS and PFOA [4]. PFAS have been found in both influent and effluent of wastewater treatment plants which are considered as one of the major sources for their occurrence in surface and groundwater [5,6,7][5][6][7]. Like several other micropollutants, PFAS are found at very low concentrations [8], but their refractory nature and unique physicochemical properties (Table 1) exacerbates the challenge of their degradation and/or removal.
| Property | PFOA (Free Acid) | PFOS (Potassium Salt) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| * Physical description | White powder/waxy white solid | White powder PFOA | |||||||||||
| Molecular formula | C | 8 | HF | 15 | O | 2 | C | 8 | HF | 17 | O | 3 | S |
| Molecular weight (g mol | −1 | ) | 414 | 538 | |||||||||
| Water solubility at 25 °C (mg L | −1 | ) | 9.5 × 10 | 3 | 680 | ||||||||
| Melting Point (°C) | 45–50 | >400 | |||||||||||
| Boiling point (°C) | 189–192 | Not measurable | |||||||||||
| Vapour pressure at 25 °C (Pa) | 4.2 | 2.48 × 10 | –8 | ||||||||||
| Organic–carbon partition coefficient (log K | oc | ) | 2.06 | ** 2.57 | |||||||||
| Henry’s law constant (atm-m | 3 | mol | −1 | ) | Not measurable | 3.05 × 10 | –9 | ||||||
| Half-Life | *** 90 days, **** >92 years (at 25 °C) | *** 114 days, **** >41 years (at 25 °C) |
* At room temperature and atmospheric pressure, ** Value estimated based on anion and not the salt, *** Atmospheric, **** In water.
2. Photolysis and Photochemical Decomposition Using UVC/VUV
2.1. Direct Photolysis (UV/VUV)
UV irradiation is divided into four regions: UVA (315–400 nm), UVB (280–315 nm), UVC (200–280 nm) and vacuum UV (VUV, 100–200 nm) [18][10]. Low pressure (LP) mercury lamps emitting UVC irradiation primarily at 254 nm have been investigated for the degradation of various contaminants including PFAS. While it is an efficient process for a range of compounds, conventional direct photolysis is known to be inefficient for the degradation PFAS as demonstrated by several investigations [19,20,21,22][11][12][13][14]. Little to no degradation during direct UV photolysis is attributed to insufficient breakdown of the C-F bond by photo energy generated during UV irradiation [23][15]. Giri et al. [23][15] corroborated these results and found negligible degradation of PFOA after 5 h of irradiation at 254 nm. The absorption of UV light at wavelength higher than 220 nm is very low resulting in direct photolysis being ineffective for PFAS degradation [24][16]. Similarly, Chen et al. [20][12] observed that the UV absorbance of PFOA from 190 to 280 nm (UVC) and reported a much stronger UV absorption in the VUV region below 200 nm.
Giri et al. [23][15] calculated the photon energy for both UVC (471.1 kJ mol−1) and VUV (185 nm) (646.8 kJ mol−1) and considering the C-C bond energy (347 kJ mol−1), PFOA is prone to breakage by both wavelengths. However, C-F bond with the higher bond energy (552 kJ mol−1) is unlikely to be cleaved by 254 nm. It is therefore established that direct UV photolysis at 254 nm is not a suitable process for the degradation of these compounds. Considering the higher photon energy generated during VUV photolysis, direct photolysis by VUV is more promising.
Although there are limited studies using VUV irradiation, the findings suggest that the process has the potential to degrade PFAS. Considering stronger UV absorption below 200 nm, the application of VUV photolysis requires to be investigated further using specially designed system emitting most irradiation at 185 nm such as in the case of Giri et al. [23][15] who reported 97% VUV emission when used a synthetic fused silica glass tube. It is worth noting that the VUV process generates ozone that could also lead to the degradation of PFAS. However, none of the studies have looked at the generation and potential degradation of PFAS related to ozone produced during VUV process. Furthermore, it has been previously demonstrated that the generation of H2O2 is enhanced during UV/VUV process in the presence of oxygen but was negatively impacted under alkaline conditions and in the presence of anions [25][17]. The addition of chemicals could therefore be avoided during VUV process due to in-situ generation H2O2 [26,27][18][19]. Hence, it is important that VUV process is investigated with a particular focus given to optimizing conditions for greater generation of H2O2. The role of ozone generated during VUV process also needs to be investigated.
2.2. Photochemical Oxidation Using UVC/VUV
2.2.1. UV/H2O2
One of the most commonly used UV-based advanced oxidation processes (AOPs) that has been investigated for the degradation of recalcitrant organic contaminants is UV/H2O2. However, the addition of H2O2 is found to be unfavorable for the degradation of PFOA due to competitive absorption of photons [28][20]. The upper limit for the second-order rate constant of •OH reaction with PFOA (k•OH + PFOA) is ≤105 L·mol−1·s−1, which is several orders of magnitude slower than the •OH reaction with most hydrocarbons [29][21]. The reaction of •OH with organic compounds principally involves three mechanisms: (1) hydrogen abstraction yielding carbon-centered radicals, (2) electrophilic addition of •OH to unsaturated carbon-carbon bonds, and (3) electron transfer in which case the •OH receives an electron from the organic substituent [30][22]. Reaction of •OH with saturated organic compounds occurs through hydrogen abstraction and unsaturated organic compounds via radical addition. Since PFOA and PFOS has no hydrogen for abstraction, •OH can only react via direct electron transfer pathway leading to formation of much less thermodynamically favored HO− (E0 = 1.9 V). The perfluorination or substitution of organic hydrogen atoms for fluorines make the PFASs inert to •OH oxidation [31][23]. Convectional UV/H2O2 process is therefore not considered efficient for the degradation of PFOA and PFOS. This has prompted research into using other oxidative agents in combination with UV irradiation.
2.2.2. UV/VUV/Sulfite
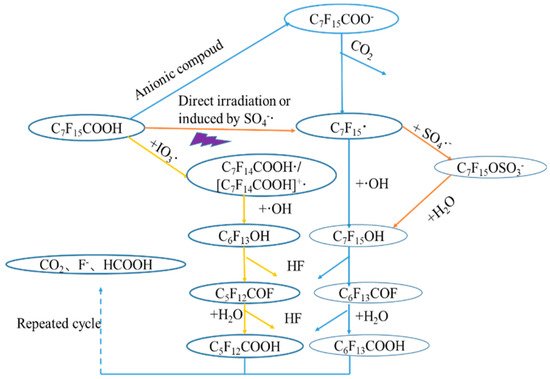
A comparison between VUV alone and in combination with K2S2O8 and Na2S under oxygen and nitrogen atmosphere, respectively, was made for the degradation of perfluorodecanoic acid (PFDeA) [40][24]. The degradation of PFDeA was ~60% after 5 h irradiation (light intensity of 62–69 mW cm−2) but a much lower defluorination ratio of ~16% was achieved using VUV alone. However, in the presence of K2S2O8 under oxygen atmosphere, the concentration of PFDeA was below the detection limit (not given) with 36% defluorination after 5 h. Increasing the K2S2O8 concentration from 0.1 mM to 5 mM did not improve the PFDeA degradation and the formation of F− that ranged between 0.41 and 0.43 mg·L−1·min−1. The trend of PFDeA degradation in the presence of 0.1–5 mM Na2S under nitrogen atmosphere was fairly similar to VUV/K2S2O8 system and the concentration of PFDeA was below the detection limit for both 0.1 and 0.5 mM Na2S. Both direct photolysis of PFDeA by VUV and photochemical decomposition by SO∙−4 SO4•− was proposed during VUV/K2S2O8 process with SO∙−4 SO4•− playing a significant role in the oxidative degradation of PFDeA. During VUV/Na2S treatment, however, both oxidative and reductive (by e−aq) degradation occurred but no conclusion regarding which mechanism was most prevalent was made requiring this system to be investigated further. The PFOA degradation pathway during UV-based AOPs including SO∙−4 O4•− is given in Figure 1 [17][25]. Briefly, PFOA degraded to fluoride ions, formic acid and CO2 via sequential removal of CF2 during UV-based AOPs.
2.3. Reaction by-Products during UV/VUV Photodegradation
Investigating PFOA degradation using VUV/Fe3+, Cheng et al. [34][26] identified intermediate by-products containing C2–C7 perfluoroalkyl groups in agreement with previous investigations [20,28][12][20]. The longer chain intermediates including PFHpA (C7) and perfluorohexanoic acid (PFHxA) (C6) reached maximum concentration after 1.5 h and 3 h, respectively, followed by decreased concentration with increasing irradiation time. The remaining intermediates (C2–C5) increased throughout the 4 h irradiation period. The order of concentration followed PFPeA > PFBA > perfluoropropionic acid (PFPA) > trifluoroacetic acid (TFA) demonstrating the longer chain intermediates appeared at the start of the reaction followed by decomposition to shorter chain products. Investigating PFOA degradation using 254 nm UV during UV/H2O2/Fe2+ process, Tang et al. [41][27] found that the degradation intermediates included the short chain perfluorocarboxylic acids containing 2, 3 4, 5 and 6 carbon atoms and fluoride ions which is in agreement with others [23,64][15][28]. Liang et al. [32][29] also identified perfluoronated carboxylic acids with 2–7 carbon atoms during VUV/Fe3+ degradation of PFOA. It was further noted that the shorter chain degradation products were higher in concentration, i.e., PFPA > PFBA > PFPeA > PFHxA > PFHA [32][29]. Jin et al. [35][30] while investigating the degradation of PFOS using UV/Fe3+ found C2–C8 PFCAs in addition to sulphate and fluoride as degradation by-products. Theses intermediates are similar to that reported in earlier studies using UV/Fenton [41][27] and VUV/Fe3+ [32][29].2.4. Photochemical Oxidation Using MP UV—Impact of Experimental Conditions
Some studies have investigated the degradation of PFAS using medium pressure UV lamps. For example, Hori et al. [24][16] investigated photochemical degradation using MP UV lamp (220–460 nm) of PFCAs containing 3–5 carbon atoms (PFPrA, PFBA, or PFPeA). They reported that the absorption of UV light was much higher for deep-UV region to 220 nm but was much lower for 220–270 nm. After 24 h of direct photolysis, 24.3% of PFPeA was degraded yielding 12.1% F−. The other two PFCAs (PFPrA, PFBA) showed lower but comparable degradation and F− yield of about 16% and <10%, respectively. Degradation of these short chain PFAS enhanced when 5 mM Fe3+ was used such that the degradation of PFPeA, PFBA and PFPrA was about 2.7-, 3- and 3.8-fold greater when compared with UV alone after 24 h. Similarly, the amount of F− yield was greater although it did not follow the trend of degradation. The degradation followed pseudo-first-order kinetics with increasing rate of degradation with increasing initial concentration of PFPeA demonstrating that the complexes formed between Fe3+ and PFPeA resulted in photo-redox reactions that led to the formation of Fe2+ and oxidized PFPeA. The degradation of PFPeA was markedly higher in the presence of oxygen (64.5%) than argon (35.6%) and so was the conversion of Fe3+ to Fe2+, i.e., 93.3% and 0.70%, respectively. It was concluded that oxygen was required for re-oxidation step of Fe2+ conversion to Fe3+ since the presence of oxygen could increase the formation of HO2 that can expedite the re-oxidation process.References
- Baran, J.R. Fluorinated Surfactants and Repellents: 2nd Edition. Revised and Expanded Surfactant Science Series; American Chemical Society: Washington, DC, USA, 2001; Volume 123, p. 8882.
- Brooke, D.; Footitt, A.; Nwaogu, T.A. Environmental Risk Evaluation Report: Perfluorooctanesulphonate (PFOS); Environment Agency: Bristol, UK, 2004.
- Fujii, S.; Polprasert, C.; Tanaka, S.; Hong Lien, N.P.; Qiu, Y. New POPs in the water environment: Distribution, bioaccumulation and treatment of perfluorinated compounds—A review paper. J. Water Supply Res. Technol. Aqua 2007, 56, 313–326.
- Du, Z.; Deng, S.; Bei, Y.; Huang, Q.; Wang, B.; Huang, J.; Yu, G. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—A review. J. Hazard. Mater. 2014, 274, 443–454.
- Ahrens, L.; Barber, J.L.; Xie, Z.; Ebinghaus, R. Longitudinal and Latitudinal Distribution of Perfluoroalkyl Compounds in the Surface Water of the Atlantic Ocean. Environ. Sci. Technol. 2009, 43, 3122–3127.
- Gebbink, W.A.; van Asseldonk, L.; van Leeuwen, S.P.J. Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water near a Fluorochemical Production Plant in the Netherlands. Environ. Sci. Technol. 2017, 51, 11057–11065.
- Gomez-Ruiz, B.; Gómez-Lavín, S.; Diban, N.; Boiteux, V.; Colin, A.; Dauchy, X.; Urtiaga, A. Efficient electrochemical degradation of poly- and perfluoroalkyl substances (PFASs) from the effluents of an industrial wastewater treatment plant. Chem. Eng. J. 2017, 322, 196–204.
- Ahrens, L. Polyfluoroalkyl compounds in the aquatic environment: A review of their occurrence and fate. J. Environ. Monit. 2011, 13, 20–31.
- Espana, V.A.A.; Mallavarapu, M.; Naidu, R. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA): A critical review with an emphasis on field testing. Environ. Technol. Innov. 2015, 4, 168–181.
- Oppenländer, T. Photooxidation and Photomineralization of Organic Matter in Water and Air. In Photochemical Purification of Water and Air; Wiley: New York, NY, USA, 2002; pp. 189–238.
- Chen, J.; Zhang, P. Photodegradation of perfluorooctanoic acid in water under irradiation of 254 nm and 185 nm light by use of persulfate. Water Sci. Technol. 2006, 54, 317–325.
- Chen, J.; Zhang, P.-Y.; Liu, J. Photodegradation of perfluorooctanoic acid by 185 nm vacuum ultraviolet light. J. Environ. Sci. 2007, 19, 387–390.
- Giri, R.R.; Ozaki, H.; Morigaki, T.; Taniguchi, S.; Takanami, R. UV photolysis of perfluorooctanoic acid (PFOA) in dilute aqueous solution. Water Sci. Technol. 2011, 63, 276–282.
- Thi, L.-A.P.; Do, H.-T.; Lee, Y.-C.; Lo, S.-L. Photochemical decomposition of perfluorooctanoic acids in aqueous carbonate solution with UV irradiation. Chem. Eng. J. 2013, 221, 258–263.
- Giri, R.R.; Ozaki, H.; Okada, T.; Taniguchi, S.; Takanami, R. Factors influencing UV photodecomposition of perfluorooctanoic acid in water. Chem. Eng. J. 2012, 180, 197–203.
- Hori, H.; Yamamoto, A.; Koike, K.; Kutsuna, S.; Osaka, I.; Arakawa, R. Photochemical decomposition of environmentally persistent short-chain perfluorocarboxylic acids in water mediated by iron(II)/(III) redox reactions. Chemosphere 2007, 68, 572–578.
- Du, J.; Wang, C.; Zhao, Z.; Cui, F.; Ou, Q.; Liu, J. Role of oxygen and superoxide radicals in promoting H2O2 production during VUV/UV radiation of water. Chem. Eng. Sci. 2021, 241, 116683.
- Barki, D.; Sabach, S.; Dubowski, Y. Removal of Chlorinated Organic Pollutants from Groundwater Using a Vacuum-UV-Based Advanced Oxidation Process. ACS EST Water 2021, 1, 2076–2086.
- Yang, Y.; Zhang, Q.; Chen, B.; Long, L.; Zhang, G. Toward better understanding vacuum ultraviolet—iodide induced photolysis via hydrogen peroxide formation, iodine species change, and difluoroacetic acid degradation. Front. Environ. Sci. Eng. 2022, 16, 55.
- Hori, H.; Hayakawa, E.; Einaga, H.; Kutsuna, S.; Koike, K.; Ibusuki, T.; Kiatagawa, H.; Arakawa, R. Decomposition of Environmentally Persistent Perfluorooctanoic Acid in Water by Photochemical Approaches. Environ. Sci. Technol. 2004, 38, 6118–6124.
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886.
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698.
- Schröder, H.F.; Meesters, R.J. Stability of fluorinated surfactants in advanced oxidation processes—A follow up of degradation products using flow injection–mass spectrometry, liquid chromatography–mass spectrometry and liquid chromatography–multiple stage mass spectrometry. J. Chromatogr. A 2005, 1082, 110–119.
- Wang, B.; Cao, M.; Tan, Z.; Wang, L.; Yuan, S.; Chen, J. Photochemical decomposition of perfluorodecanoic acid in aqueous solution with VUV light irradiation. J. Hazard. Mater. 2010, 181, 187–192.
- Wang, X.; Chen, Z.; Wang, Y.; Sun, W. A review on degradation of perfluorinated compounds based on ultraviolet advanced oxidation. Environ. Pollut. 2021, 291, 118014.
- Cheng, J.-H.; Liang, X.-Y.; Yang, S.-W.; Hu, Y.-Y. Photochemical defluorination of aqueous perfluorooctanoic acid (PFOA) by VUV/Fe3+ system. Chem. Eng. J. 2014, 239, 242–249.
- Tang, H.; Xiang, Q.; Lei, M.; Yan, J.; Zhu, L.; Zou, J. Efficient degradation of perfluorooctanoic acid by UV–Fenton process. Chem. Eng. J. 2012, 184, 156–162.
- Wang, Y.; Zhang, P. Effects of pH on photochemical decomposition of perfluorooctanoic acid in different atmospheres by 185nm vacuum ultraviolet. J. Environ. Sci. 2014, 26, 2207–2214.
- Liang, X.; Cheng, J.; Yang, C.; Yang, S. Factors influencing aqueous perfluorooctanoic acid (PFOA) photodecomposition by VUV irradiation in the presence of ferric ions. Chem. Eng. J. 2016, 298, 291–299.
- Jin, L.; Zhang, P.; Shao, T.; Zhao, S. Ferric ion mediated photodecomposition of aqueous perfluorooctane sulfonate (PFOS) under UV irradiation and its mechanism. J. Hazard. Mater. 2014, 271, 9–15.
More