Infectious bronchitis virus (IBV) is the causative agent of multi-systemic infection in the respiratory, reproductive and renal systems, which is similar to the symptoms of various viral and bacterial diseases reported in chickens. Currently, the live attenuated and killed vaccines are applied for the control of IBV infection; however, the continual emergence of IB variants with rapidly evolving genetic variants increases the risk of outbreaks in intensive poultry farms.
- infectious bronchitis virus
- vaccination
- immune system
1. Introduction
2. Vaccination
| Name of the Vaccine | Route of Delivery | Characteristics | |
|---|---|---|---|
| 1. Live attenuated IBV or | Aero nasal spray | Serial attenuation of virulent IB strain for weakened | |
| Live IBV vaccines | In Ovo route | virulence [32,33]. | virulence [16][17]. |
| Orally | |||
| Subcutaneous (S/C) | |||
| 2. Killed or inactivated | IM injection | Inactivated by chemical treatment or heat treat to kill the | |
| IB vaccines | S/C | virulence of strain [34]. | virulence of strain [18]. |
| 3. Viral Vector vaccine | In ovo route | Recombinant rNDV/APMV-2 expressing the S protein of | |
| IBV strain Mass-41 (rNDV/APMV-2/IBV-S) [35]. | IBV strain Mass-41 (rNDV/APMV-2/IBV-S) [19]. | ||
| 4. DNA vaccine | Mucosal/Orally | IBV-DNA vaccine carrying S1-protein and/or N-protein constructs | |
| IM injection | the respective vector [36,37,38,39]. | the respective vector [20][21][22][23]. | |
| Intranasal | |||
| In ovo route | |||
| 5. Recombinant protein (sub-unit) |
Intraocular-nasally IM injection |
Water-in-oil emulsified recombinant S-ectodomain protein [40]. | Water-in-oil emulsified recombinant S-ectodomain protein [24]. |
| Second heptad repeat (HR2) region of S protein were | |||
| repeatedly co-displayed in the Self-assembling | |||
| Protein Nanoparticle (SAPN) [41]. | Protein Nanoparticle (SAPN) [25]. | ||
| 6. Multi-epitope-based | Oral | Using attenuated S enterica serovar Typhimurium strain [42]. | Using attenuated S enterica serovar Typhimurium strain [26]. |
| peptide vaccine | Mucosal | Recombinant DNA: The EpiC gene was presented in | |
| (Lactococcus lactis bacterial system) |
Intranasal | Lactococcus lactis NZ3900 with 3 recombinant strains expressing EpiC gene [43]. | Lactococcus lactis NZ3900 with 3 recombinant strains expressing EpiC gene [27]. |
| 7. VLP-based IBV vaccine or | IM immunized | Efficient mucosal immune response [44] | Efficient mucosal immune response [28] |
| chimeric VLP vaccine |
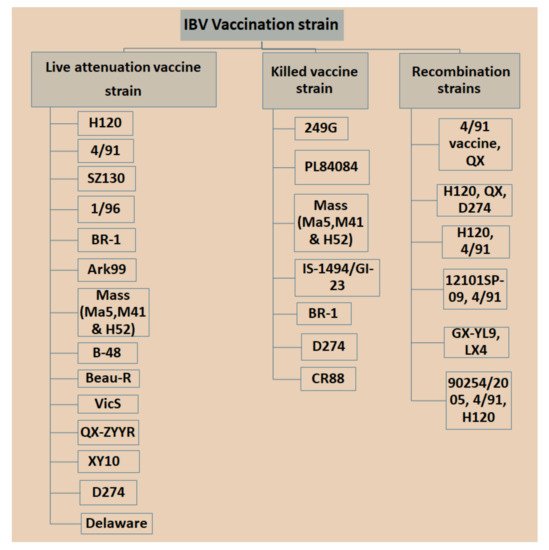
Currently, IBV vaccination programs have gained more attention with respect to the use of low-virulent, live or inactivated killed vaccines with the aim of booster shots at certain times to increase immunity and reduce the antagonistic response of epithelial cells in the respiratory region [89][29]. However, there is a significant limitation in applying live IBV vaccines because the attenuation of the vaccine is naturally deficient with respect to its capability in stimulating a mucosal immune response [90[30][31],91], which is a critical part of controlling IBV infection since killed vaccine can be an option [92,93][32][33]. Nonetheless, it was possible that inactivated killed vaccines can be applied to stimulate t mucosal immune responses once combined with several nanoparticles [92][32]. Different types of IBV vaccines are available in the market, which may vary in vaccine strains, and in nature based on local isolate and recombination in strains isolated from different countries with special legal legislation (Figure 31). Bijlenga et al. [49][34] described the earlier development of the H strain of IBV containing both the H52 and H120 due to its better capability of heterologous cross-protection against different serotypes of IBV. Further studies have revealed that heterologous IBV vaccines are also more effective for immunizing the 793B-type of variant that has been evidenced to be long lasting with live attenuated IB vaccines and are effectively applied against Italy 02 and QX stain [94,95,96][35][36][37]. The modified live vaccines and inactivated oil emulsions are available for a few serotypes such as Massachusetts, Connecticut and Arkansas in North America. The California strains and Georgia 98 vaccines are collected from the USA.
3. Immune Response against IBV
3.1. Local Immune System
A vaccine requires a certain period of time in order to elicit a protective immune response in avian hosts. Moreover, passive immunization can induce immunity from maternal derivative’s antibody (MDA), which is particularly supportive during the early stages of life [106][43]. The structure and function of birds’ immune systems are distinctly different from human immune systems due to their virtue of possessing extra lymphatic organs such as the bursa of Fabricius and the thymus responsible for humoral and cellular immunity, respectively. Furthermore, the birds have carried the secondary peripheral organs of the lymphatic system, for example, the Harderian gland (HG), conjunctiva associated lymphoid tissue (CALT), head associated lymphoid tissue (HALT), gut-associated lymphoid tissue (GALT) and bronchus associated lymphoid tissue (BALT), spleen and cecum tonsils, respectively [107][44], showed in Figure 42. These assemblies are regularly enmeshed in a chicken’s immune response, especially in the respiratory mucosal system during IBV infection.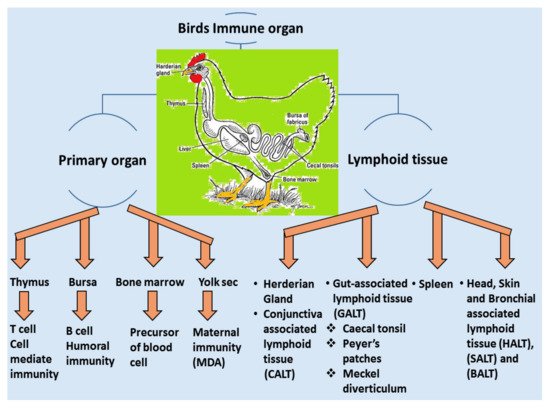
3.2. Adaptive Immune System
3.2.1. Humoral Immunity
3.2.2. Cell-Mediated Immunity (CMI)
In chickens, CMI is one of the essential immunoregulatory weapons during IBV infection, especially for aiding the clearance of viruses, decline of infection and reducing virus shedding and vaccine development [146][49]. The evaluation of cellular arms is performed by lymphocytic transformation assays, cytotoxic lymphocyte activity [147][50], delayed-type hypersensitivity [148][51] and natural killer cell activity [149][52]. Histological lesions of CMI responses are performed by T-cell infiltration in the respiratory and renal tissues of IBV-infected chickens [150][53]. The experimental studies have shown a positive relationship between lymphoproliferative responses and resistance to challenge infection [39][23]. Alternative studies have been published on mouse monoclonal antibodies (mAbs) that differentiate between T-lymphocytes and are used to evaluate the role of T-cells in viral clearance [151][54]. N and S genes have a specific protein response associated with the stimulated virus-specific protective immunity of CTLs, which is characterized by the reduction in viral load and clearance of the virus from circulation [152,153][55][56]. A marked increase in CD4+ and CD8+ T-cells has been described as the recombinant S1-gene associated with the induction of cellular immunity of specific IBV vaccines [154][57]. Guo et al. [155][58] reported that IB vaccination with N gene-based DNA vaccine significantly increased the number of CD4+ and CD8 + T cells in peripheral blood mononuclear cells (PBMCs). The existence of CD8+ cytotoxic T lymphocytes (CTL) signifies an essential relationship for reducing infection and resembles a decrease in clinical signs by the action of major histocompatibility complex (MHC), and lysis is facilitated by CD8+CD4 cells [156][59]. Consequently, the major histocompatibility complex organized cytotoxic T lymphocytes (CTL), and the cytokine activities of chickens participated during the early stages of IBV infections [157][60]. Several studies have been conducted on tracheal immunity induced by live vaccines, and they found that all vaccines induced significantly higher expression of CD4+ and CD8+ compared with unvaccinated birds using a nephron-pathogenic IBV strain [44][28]. In the following year, other studies have reported that CD4+ cells are recruited into the trachea earlier than CD8+ on 5 dpi (days of post-infection) [158][61], which agrees with the findings by Kotani et al. [159][62] who recognized that the frequency of CD4+ and CD8+ cell numbers significantly peaked at 5 dpi when using a virulent IBV strain. In contrast, studies reported that CD8+ cells were recruited into the trachea earlier than CD4+ cells after infection with virulent 793B [160][63] or live attenuated IBV vaccine [44][28] or a combination of live attenuated vaccine with a booster dose of an inactivated vaccine [161][64]. CD8+ memory T cells have greatly protected the newborn chicks from acute IBV infection at 4 dpi and mild clinical symptoms show at 5 dpi [156][59]. Even though the adoptive transfer of CD4 + T cells could not be significantly protected in the early stage of IBV infection, primed αβ T cells carrying CD8+ T cells are critical in protecting chicks from IBV infection [152][55]. Chhabra et al. [44][28] reported that the protection against Q1-IBV strain changes the quantity of CD4+ and CD8+ cells in the trachea using immunohistochemistry. The results showed that the overall patterns of CD8+ cells are dominant compared to those of CD4+ cells in the two vaccinated groups. The kinetics of CD4+, CD8+ and the IgA-carrying B lymphocytes in the trachea are shown in Figure 53B–D) compared with control Figure 53A in vaccinated groups as differences may have a close relationship with the IBV-specific strains.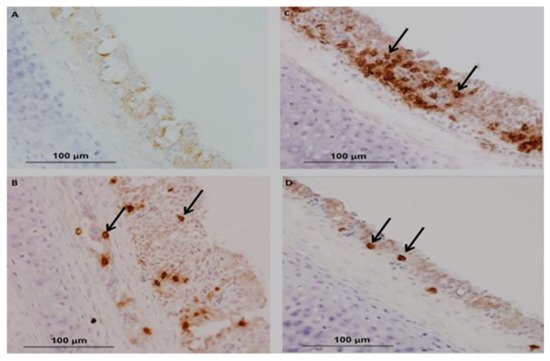
References
- Zhang, X.; Liao, K.; Chen, S.; Yan, K.; Du, X.; Zhang, C.; Guo, M.; Wu, Y. Evaluation of the reproductive system development and egg-laying performance of hens infected with TW I-type infectious bronchitis virus. Vet. Res. 2020, 51, 95.
- He, K.; Li, M.; Wei, P.; Mo, M.L.; Wei, T.C.; Li, K.R. Complete genome sequence of an infectious bronchitis virus chimera between cocirculating heterotypic strains. J. Virol. 2012, 86, 13887–13888.
- Cavanagh, D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007, 38, 281–297.
- Cook, J.K.A.; Jackwood, M.; Jones, R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012, 41, 239–250.
- Gay, K. Infectious Bronchitis Virus Detection and Persistence in Experimentally Infected Chickens. Master’s Thesis, Cornell University, Ithaca, NY, USA, 2000.
- Legnardi, M.; Tucciarone, C.M.; Franzo, G.; Cecchinato, M. Infectious Bronchitis Virus Evolution, Diagnosis and Control. Vet. Sci. 2020, 7, 79.
- Brian, D.; Baric, R. Coronavirus genome structure and replication. In Coronavirus Replication and Reverse Genetics; Brian, D.A., Baric, R.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–30.
- Cavanagh, D.; Mawditt, K.; Welchman, D.d.B.; Britton, P.; Gough, R. Coronaviruses from pheasants (Phasianus colchicus) are genetically closely related to coronaviruses of domestic fowl (infectious bronchitis virus) and turkeys. Avian Pathol. 2002, 31, 81–93.
- Papineau, A.; Berhane, Y.; Wylie, T.N.; Wylie, K.M.; Sharpe, S.; Lung, O. Genome organization of Canada Goose Coronavirus, a novel species identified in a mass die-off of Canada Geese. Sci. Rep. 2019, 9, e5954.
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23.
- Alluwaimi, A.M.; Alshubaith, I.H.; Al-Ali, A.M.; Abohelaika, S. The Coronaviruses of Animals and Birds: Their Zoonosis, Vaccines, and Models for SARS-CoV and SARS-CoV2. Front. Vet. Sci. 2020, 7, 582287.
- Elengoe, A. COVID-19 Outbreak in Malaysia. Osong Public Health Res. Perspect. 2020, 11, 93–100.
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154.
- Khan, S.; Siddique, R.; Shereen, M.A.; Ali, A.; Liu, J.; Bai, Q.; Bashir, N.; Xue, M. Emergence of a Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2: Biology and Therapeutic Options. J. Clin. Microbiol. 2020, 58, e00187-20.
- Siddell, S.; Snijder, E.J. An introduction to Nidoviruses. In Nidoviruses; Perlman, S., Gallagher, T., Snijder, E., Eds.; ASM Press: Washington, DC, USA, 2008; pp. 1–14.
- Sultan, H.A.; Ali, A.; El Feil, W.K.; Bazid, A.H.I.; El-Abideen, M.A.Z.; Kilany, W.H. Protective Efficacy of Different Live Attenuated Infectious Bronchitis Virus Vaccination Regimes Against Challenge With IBV Variant-2 Circulating in the Middle East. Front. Vet. Sci. 2019, 9, 341.
- Guzmán, M.; Hidalgo, H. Live Attenuated Infectious Bronchitis Virus Vaccines in Poultry: Modifying Local Viral Populations Dynamics. Animals 2020, 10, 2058.
- Jackwood, M.W.; Hilt, D.A.; Sellers, H.S.; Williams, S.M.; Lasher, H.N. Rapid heat-treatment attenuation of infectious bronchitis virus. Avian Pathol. 2010, 39, 227–233.
- Shirvani, E.; Samal, S.K. Comparative Protective Efficacies of Novel Avian Paramyxovirus-Vectored Vaccines against Virulent Infectious Bronchitis Virus in Chickens. Viruses 2020, 12, 697.
- Babapoor, S.; Almeida, D.d.O.; Fabis, J.J.; Helal, Z.H.; Wang, X.; Girshick, T.; Khan, M.I. Protective effect of In ovo vaccination with IBV-spike-recombinant DNA and chicken interferon as an adjuvant. Int. J. Poult. Sci. 2009, 11, 1034–1041.
- Tan, L.; Zhang, Y.; Liu, F.; Yuan, Y.; Zhan, Y.; Sun, Y.; Qui, X.; Meng, C.; Song, C.; Ding, C. Infectious bronchitis virus poly-epitope-based vaccine protects chickens from acute infection. Vaccine 2016, 34, 5209–5216.
- Zuo, L.; Yan, W.; Song, Z.; Li, H.; Xie, X.; Gu, K.; Ma, P.; Tian, Y.; Zhou, C.; Zhao, Y.; et al. Design and Characterization of a DNA Vaccine Based on Spike with Consensus Nucleotide Sequence against Infectious Bronchitis Virus. Vaccines 2021, 9, 50.
- Yan, F.; Zhao, Y.; Hu, Y.; Qiu, J.; Lei, W.; Ji, W.; Li, X.; Wu, Q.; Shi, X.; Li, Z. Protection of chickens against infectious bronchitis virus with a multivalent DNA vaccine and boosting with an inactivated Vaccine. J. Vet. Sci. 2013, 14, 53–60.
- Eldemery, F.; Joiner, K.S.; Toro, H.; van Santen, V.L. Protection against infectious bronchitis virus by spike ectodomain subunit Vaccine. Vaccine 2017, 35, 5864–5871.
- Li, J.; Helal, Z.H.; Karch, C.P.; Mishra, N.; Girshick, T.; Garmendia, A.; Burkhard, P.; Khan, M.I. A self-adjuvanted nanoparticle based vaccine against infectious bronchitis virus. PLoS ONE 2018, 13, e0203771.
- Jiao, H.; Pan, Z.; Yin, Y.; Geng, S.; Sun, L.; Jiao, X. Oral and nasal DNA vaccines delivered by attenuated Salmonella enterica serovar Typhimurium induce a protective immune response against infectious bronchitis in chickens. Clin. Vaccine Immunol. 2011, 18, 1041–1045.
- Cao, H.P.; Wang, H.N.; Yang, X.; Zhang, A.Y.; Li, X.; Ding, M.D.; Liu, S.T.; Zhang, Z.K.; Yang, F. Lactococcus lactis anchoring avian infectious bronchitis virus multi-epitope peptide EpiC induced specific immune responses in chickens. Biosci. Biotechnol. Biochem. 2013, 77, 1499–1504.
- Chhabra, R.; Forrester, A.; Lemiere, S.; Awad, F.; Chantrey, J.; Ganapathy, K. Mucosal, Cellular, and Humoral Immune Responses Induced by Different Live Infectious Bronchitis Virus Vaccination Regimes and Protection Conferred against Infectious Bronchitis Virus Q1 Strain. Clin. Vaccine Immunol. 2015, 22, 1050–1059.
- Kapczynski, D.R.; King, D.J. Protection of chickens against overt clinical disease and determination of viral shedding following vaccination with commercially available Newcastle disease virus vaccines upon challenge with highly virulent virus from the California 2002 exotic Newcastle disease outbreak. Vaccine 2005, 23, 3424–3433.
- Hassan, K.E.; El-Kady, M.F.; El-Sawah, A.A.A.; Luttermann, C.; Parvin, R.; Shany, S.; Beer, M.; Harder, T. Respiratory disease due to mixed viral infections in poultry flocks in Egypt between 2017 and 2018: Upsurge of highly pathogenic avian influenza virus subtype H5N8 since 2018. Transbound. Emerg. Dis. 2019, 68, 21–36.
- Ball, C.; Bennett, S.; Forrester, A.; Ganapathy, K. Genetic mutations in live infectious bronchitis vaccine viruses following single or dual in vitro infection of tracheal organ cultures. J. Gen. Virol. 2016, 97, 3232–3237.
- Lopes, P.D.; Okino, C.H.; Fernando, F.S.; Pavani, C.; Casagrande, V.M.; Lopez, R.F.V.; Montassier, M.d.F.S.; Montassier, H.J. Inactivated infectious bronchitis virus vaccine encapsulated in chitosan nanoparticles induces mucosal immune responses and effective protection against challenge. Vaccine 2018, 36, 2630–2636.
- Jordan, B. Vaccination against infectious bronchitis virus: A continuous challenge. Vet. Microbiol. 2017, 206, 137–143.
- Bijlenga, G.; Cook, J.K.A.; Gelb, J., Jr.; de Wit, J.J. Development and use of the H strain of avian infectious bronchitis virus from the Netherlands as a vaccine: A review. Avian Pathol. 2004, 33, 550–557.
- Cook, J.K.A.; Orbell, S.J.; Woods, M.A.; Huggins, M.B. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 1999, 28, 477–485.
- Jones, R.C.; Worthington, K.J.; Capua, I.; Naylor, C.J. Efficacy of live infectious bronchitis vaccines against a novel European genotype, Italy 02. Vet. Rec. 2005, 156, 646–647.
- De Wit, J.J.; Van de Sande, H.; Prandini, F. Enhanced efficacy of the use of a monovalent infectious bronchitis virus inactivated vaccine in layers primed with H120 and 793B live IBV vaccines to increase the protection against challenge with 3 European serotypes of IBV. In Proceedings of the VI International Symposium on Corona- and Pneumoviruses and Complicating Pathogens, Rauischholzhausen, Germany, 14–17 June 2009; pp. 159–167.
- Erf, G.F. Cell-mediated immunity in poultry. Poult. Sci. 2004, 83, 580–590.
- Qureshi, M.A.; Heggen, C.L.; Hussain, I. Avian macrophage: Effector functions in health and disease. Dev. Comp. Immunol. 2000, 24, 103–119.
- Berghman, L.R. Immune responses to improving welfare. Poult. Sci. 2016, 95, 2216–2218.
- Jansen, C.A.; van de Haar, P.M.; van Haarlem, D.; van Kooten, P.; de Wit, S.; van Eden, W.; Viertlböck, B.C.; Göbel, T.W.; Vervelde, L. Identification of new populations of chicken natural killer (NK) cells. Dev. Comp. Immunol. 2010, 34, 759–767.
- Sharma, R.K.; Yolcu, E.S.; Srivastava, A.K.; Shirwan, H. CD4+ T cells play a critical role in the generation of primary and memory antitumor immune responses elicited by SA-4-1BBL and TAA-based vaccines in mouse tumor models. PLoS ONE 2013, 8, e73145.
- Niewiesk, S. Maternal antibodies: Clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front. Immunol. 2014, 5, 446.
- Rumińska, E.; Koncicki, A.; Stenzel, T. Structure and function of the avian immune system in birds. Medycyna Wet. 2008, 64, 265–268.
- Ruano, M.; El-Attrache, J.; Villegas, P. A rapid-plate hemagglutination assay for the detection of infectious bronchitis virus. Avian Dis. 2000, 44, 99–104.
- Adler, L.N.; Jiang, W.; Bhamidipati, K.; Millican, M.; Macaubas, C.; Hung, S.C.; Mellins, E.D. The Other Function: Class II-Restricted Antigen Presentation by B Cells. Front. Immunol. 2017, 8, 319.
- Najimudeen, S.M.; Hassan, M.S.H.; Cork, S.C.; Abdul-Careem, M.F. Infectious Bronchitis Coronavirus Infection in Chickens: Multiple System Disease with Immune Suppression. Pathogens 2020, 9, 17.
- Ghadakchi, H.; Dadras, H.; Pourbakhsh, S.A.; Hosseini, S.M.H. Standardization of an Enzyme-Linked Immunosorbent Assay for Detection of Infectious Bronchitis Virus Antibody. Arch. Razi Inst. 2005, 59, 75–83.
- Okino, C.H.; dos Santos, I.L.; Fernando, F.S.; Alessi, A.C.; Wang, X.; Montassier, H.J. Inflammatory and cell-mediated immune responses in the respiratory tract of chickens to infection with avian infectious bronchitis virus. Viral Immunol. 2014, 27, 383–391.
- Chubb, R.C.; Huynh, V.; Bradley, R. The induction and control of delayed type hypersensitivity reactions induced in chickens by infectious bronchitis virus. Avian Pathol. 1988, 17, 371–383.
- Chubb, R.C.; Huynh, V.; Law, R. The detection of cytotoxic lymphocyte activity in chickens infected with infectious bronchitis virus or fowlpox virus. Avian Pathol. 1987, 16, 395–405.
- Thompson, G.; Naqi, S. Cytotoxic activity of cells recovered from the respiratory tracts of chickens inoculated with infectious bronchitis virus. Avian Dis. 1997, 41, 690–694.
- Okino, C.H.; Mores, M.A.Z.; Trevisol, I.M.; Coldebella, A.; Montassier, H.J.; Brentano, L. Early immune responses and development of pathogenesis of avian infectious bronchitis viruses with different virulence profiles. PLoS ONE 2017, 12, e0172275.
- Chen, H.D.; Fraire, A.E.; Joris, I.; Welsh, R.M.; Selin, L.K. Specific history of heterologous virus infections determines antiviral immunity and immunopathology in the lung. Am. J. Pathol. 2003, 163, 1341–1355.
- Seo, H.S.; Pei, J.; Briles, W.E.; Dzielawa, J.; Collisson, E.W. Adoptive transfer of infectious bronchitis virus primed alphabeta T cells bearing CD8 antigen protects chicks from acute infection. Virology 2000, 32, 183–189.
- Samanta, D.; Park, Y.; Ni, X.; Li, H.; Zahnow, C.A.; Gabrielson, E.; Pan, F.; Semenza, G.L. Chemotherapy induces enrichment of CD47+ /CD73+ /PDL1+ immune evasive triple-negative breast cancer cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1239–E1248.
- Meir, R.; Krispel, S.; Simanov, L.; Pitcovski, J. Immune Responses to Mucosal Vaccination by the Recombinant S1 and N Proteins of Infectious Bronchitis Virus. Viral Immunol. 2012, 25, 55–62.
- Guo, Z.; Wang, H.; Yang, T.; Wang, X.; Lu, D.; Li, Y.; Zhang, Y. Priming with a DNA vaccine and boosting with an inactivated vaccine enhance the immune response against infectious bronchitis virus. J. Virol. Methods 2010, 167, 84–89.
- Pei, J.; Briles, W.E.; Collisson, E.W. Memory T cells protect chicks from acute infectious bronchitis virus infection. Virology 2003, 306, 376–384.
- Wilson, L.; Gage, P.; Ewart, G. Hexamethylene amiloride blocks E protein ion channels and inhibits coronavirus replication. Virology 2006, 353, 294–306.
- Awad, F.; Hutton, S.; Forrester, A.; Baylis, M.; Ganapathy, K. Heterologous live infectious bronchitis virus vaccination in day-old commercial broiler chicks: Clinical signs, ciliary health, immune responses and protection against variant infectious bronchitis viruses. Avian Pathol. 2016, 45, 169–177.
- Kotani, T.; Wada, S.; Tsukamoto, Y.; Kuwamura, M.; Yamate, J.; Sakuma, S. Kinetics of lymphocytic subsets in chicken tracheal lesions infected with infectious bronchitis virus. J. Vet. Med. Sci. 2000, 62, 397–401.
- Dhinakar Raj, G.; Jones, R.C. An in vitro comparison of the virulence of seven strains of infectious bronchitis virus using tracheal and oviduct organ cultures. Avian Pathol. 1996, 25, 649–662.
- Santos, R.M.D.; Fernando, F.S.; Montassier, M.F.S.; Silva, K.R.; Lopes, P.D.; Pavani, C.; Borzi, M.M.; Okino, C.H.; Montassier, H.J. Memory immune responses and protection of chickens against a nephropathogenic infectious bronchitis virus strain by combining live heterologous and inactivated homologous vaccines. J. Vet. Med. Sci. 2019, 81, 612–619.
