Dry reforming of hydrocarbons (DRH) is a pro-environmental method for syngas production. It owes its pro-environmental character to the use of carbon dioxide, which is one of the main greenhouse gases. Transition metal carbides (TMCs) can potentially replace traditional nickel catalysts due to their stability and activity in DR processes.
- dry reforming
- catalysts
- metal carbides
- molybdenum
- tungsten
- titanium
1. Introduction
| Process | Main Reaction | Enthalpy ΔH0298 K [kJ/mol] | Pressure [bar] | H2/CO Ratio |
|---|---|---|---|---|
| Dry reforming of methane (DRM) | CH4 + CO2 = 2CO + 3 H2 | +247 | 1 | 1:1 |
| Steam reforming of methane (STM) | CH4 + H2O = CO + 3H2O | +206 | 3–25 | 3:1 |
| Partial oxidation of methane (POM) | CH4 + ½ O2 = CO + 2H2 | −35.2 | 100 | 2:1 |
| Autothermal reforming (ATR) | CH4 + H2O = CO + 3H2O CH4 + ½ O2 = CO + 2H2 |
+206 −35.2 |
1–50 | 1:1 or 1:2 |
2. Metal Carbides
2.1. Tungsten Carbide
2.2. Molybdenum Carbide
2.3. Titanium Carbide
3. The Use of Metal Carbides for Dry Reforming
3.1. Tungsten Carbide
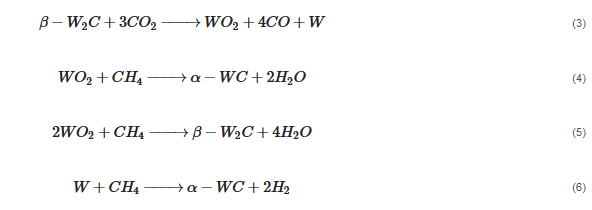
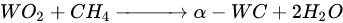

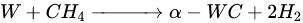


3.2. WC Combined with Nickel and Cobalt Particles
5.2. WC Combined with Nickel and Cobalt Particles
Tungsten carbide is used as a catalyst in dry methane reforming, usually in combination with nickel or cobalt, because the addition of a second metal can modify the catalytic performance and structure of this carbide [45][46][204,205]. Despite the unique properties of WC, this compound has a surface with a strong oxygen affinity. As a consequence, this leads to blockage of the surface in the event of irreversible adsorption of oxygen-containing substances, which, in turn, results in a reduction in catalytic activity [47][206]. Therefore, to avoid this problem, core–shell systems are used, that is, WC cores covered with a metallic coating that prevents oxidation of the carbide surface, thus promoting structural stability [48][207]. Co-WC and Ni-WC are stable, active, and selective catalysts in dry methane reforming [49][208].
According to Barbosa et al., higher CO2 conversion values compared to CH4 conversion in dry methane reforming are obtained using Ni-Mo2C and Ni-WC catalysts [50][209]. This is probably due to the reactions occurring, including the Boudouard reaction (Equation (79)), as a result of which the forming CO2 is activated in the carbide (Equation (810)), leading to the oxidation of WC (Equations (911) and (102)) and the conversion of CO with steam (Equation (113)), in which part of the hydrogen obtained reacts with CO2, resulting in a lower H2/CO ratio and in increased CO2 conversion. However, regardless of the presence of nickel and the Ni/W ratio, the less stable β-W2C is transformed into α-WC during dry methane reforming, according to Equations (3)–(6) [39][198].
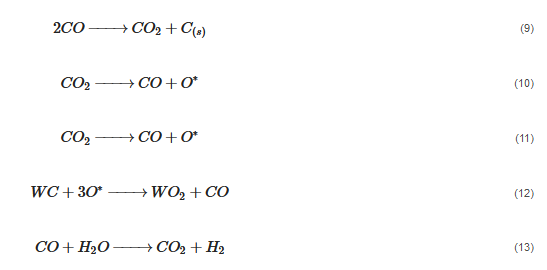
(7)
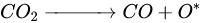
(8)
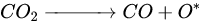
(9)

(10)

(11)
In the case of cobalt tungsten carbide (Co6W6C), the addition of carbon in the early stages of the catalytic reaction results in the conversion of the bimetallic carbide to a stable form containing active sites for dry methane reforming [46][205], according to Equation (124).

5.3. Molybdenum Carbide
(12)
3.3. Molybdenum Carbide
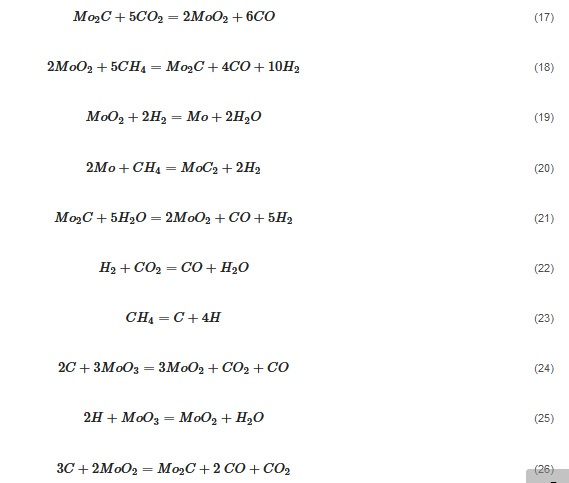
(13)
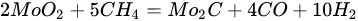
(14)
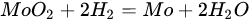
(15)
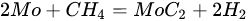
(16)
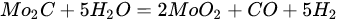
(17)
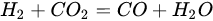
(18)
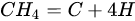
(19)
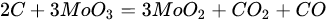
(20)
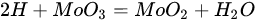
(21)
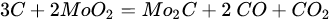
(22)
An overwhelming number of research reports on the catalytic activity of molybdenum carbides in the dry reforming of hydrocarbons refer to catalysts prepared using TPR method. The physicochemical properties and resulting catalytic activity of molybdenum carbide catalysts are influenced by the molybdenum-to-carbon ratio. Gao et al. [55][79] reported a series of molybdenum carbide catalysts that differ in the weight content of Mo in order to use carbon nanotubes as a carbon source (Mo 0, 5, 10, 15, 30, 60, and 100 wt.%). Along with an increasing proportion of molybdenum in the catalyst, a decrease in the specific surface area, diameter, and pore volume was observed. A correlation was observed between the molybdenum content and catalytic activity in the dry methane reforming process. The highest activity was observed for the catalyst containing 30 wt.% of Mo. Another of the key structural parameters of the carbide for catalytic activity is the excess unbound carbon formed during the synthesis process. Roohi et al. [56][216] found that the amount of excess carbon depends on the carburization temperature and the concentration of carbon-containing gas during the synthesis. Catalysis with lower contents of excess carbon exhibited an initial higher activity in the DRM reaction; however, during the long-term test, the molybdenum loading was a crucial factor. Several articles have been published to investigate the effect of the crystal structure on catalytic activity in DRM [57][58][78,217]. Liang et al. [57][78] investigated the catalytic activity of β-Mo2C and α-MoC1-x phases in DRM. Both phases were characterized by a narrow size distribution of up to 5 nm. Better activity was observed for the -MoC1-x phase. Oshikawa et al. [58][217] observed the dependence of the η-Mo3C2 phase on the methane decomposition rate. They reported the key role of the η-Mo3C2 phase among other molybdenum carbide phases as an active species for methane reforming. During the DRM process, the molybdenum carbide may be partially oxidized to the form of an oxycarbide. Kurlov et al. [59][218] reported that the oxycarbidic phase Mo2CxOy exhibits high stability toward further oxidation to MoO2, and the increase in β-Mo2C/ Mo2CxOy active sites correlates with higher efficiency in the DRM reaction.3.4. Molybdenum Carbide Modified with Nickel Particles
5.4. Molybdenum Carbide Modified with Nickel Particles
Molybdenum carbide catalysts during DRM at atmospheric pressure may suffer from deactivation due to oxidation with carbon dioxide. The carbide structure is reconstructed with the carbon element from the dissociation of methane; however, oxidation with CO2 is more favorable [60][219]. The combination of molybdenum carbide with other metals: Ni [61][222], Co [62][223], and Fe [63][24], allows controlled dissociation paths of CO2 and CH4, ensuring appropriate conditions for oxidation–recarburization cycles [16][64][47,224]. The introduction of other metals into the carbide catalyst results in the generation of more moles of hydrogen, leading to a higher H2/CO ratio in the outlet stream. Carbide and the introduced metal (Ni, Co) act as an active center for the dissociation of CO2 and methane, respectively. It is generally accepted that the catalytic activity of nickel catalysts is strictly connected with the size of the nickel particles: the smaller the Ni particles, the better the catalytic activity, resulting from the stronger active metal–support interactions, delayed sintering, and a lower rate of formation of carbon deposits [3][5][65][66][3,5,14,225]. However, in the case of molybdenum carbide supported nickel catalysts, the ratio of Ni/Mo to the size of nickel particles plays a predominant role [19][67][51,226]. The nickel-to-molybdenum ratio affects the morphology and catalytic activity of Mo2C. Moreover, too high a dissociation of CH4 promotes the formation of coke on the surface of the catalysts [55][61][79,222]. Zhang et al. [61][222] observed that with an increasing nickel content in nickel-modified Mo2C supported on carbon nanotubes, the crystallite size of Mo2C for Ni/Mo ratios = 0.5, 1, 1.5, and 2 was equal to 53, 38, 35, and 28 nm, respectively. Moreover, the increase in the Ni content resulted in an increase in the particle size. Catalytic activity increased with an increasing Ni/Mo ratio to the optimal value (1:1). After this value was exceeded, the activity decreased despite the higher content and smaller particle size of nickel. The DRM process is carried out mainly at temperatures above 800 °C. The performance of processes at lower temperatures results in lower methane and carbon dioxide conversions, as well as a lower H2/CO ratio [68][69][119,123]. However, Diao et al. [70][227] recently reported the high catalytic activity of a Ni-Mo2C/Al2O3 catalyst at 470 °C in a catalytic bed coupled with non-thermal plasma treatment. The molybdenum-nickel-alumina catalyst exhibited superior activity compared to Ni/Al2O3. The H2/CO ratio was equal to 0.9, and the conversions of CH4 and CO2 were around 80% and 85%, respectively. Both bare and nickel-modified molybdenum carbide catalysts are used, both supported and unsupported. Deposition on an inert substrate allows for dilution of the catalyst, thus eliminating channeling, and retarding heat transfer limitations and pressure drop across the catalytic bed [34][194]. As a support, metal oxides: La2O3 [64][224], Al2O3 [71][72][67][12,121,226], SiO2 [10], ZrO2 [73][116], MgO [74][228], biochar [75][77], carbon nanotubes [61][222], zeolites [76][26], and silicon carbide [10], have been examined. Silva et al. [10] investigated the effect of the support (SiO2, Al2O3, and SiC) Ni-Mo2C on catalytic activity and stability in the DRM reaction. The lowest DRM substrate conversions and H2/CO ratios were observed for the silica support. As a reason for the low activity observed for the SiO2-supported samples, there were weak interactions between Ni and SiO2, leading to movement of Ni species at the surface of the catalysts, retarding the interface contact between Ni and Mo2C responsible for the oxidation–recarburization cycle, Ni aggregates, and the formation of filamentous carbon.3.5. MAX and MXenes for Dry Reforming of Hydrocarbons
5.5. MAX and MXenes for Dry Reforming of Hydrocarbons
A special family of transition metal carbides is constituted by multilayer metal carbides with a 2D nanosheet structure similar to that of graphene, belonging to the group of compounds called MXenes. The term MXenes denotes carbides and nitrides of transition metals, with the general formula Mn+1XnTx, where n = 1, 2, 3, or 4, M refers to the transition metal (M = Sc, Ti, V, Cr, Mn, Y, Zr, Nb, Mo, Hf, Ta, and W [77][78][23,161]), and X refers to the p-block element (silicon, aluminum, gallium), while T describes the type of terminal groups (–O, –OH, –F, –Cl) in the amount of x per selected unit. They are obtained by selectively removing component A from the ternary MAX matrix. The MAX matrix consists of the elements of the transition metal M, a p group element (A), and carbon or nitrogen (X). MXene compounds are gaining importance due to their metal-like conductive properties, thermal and chemical stability, and the ability to manipulate properties through simple and effective modification of terminal groups [79][80][162,163]. Their unique properties allow for application in various branches of science: energy storage [80][81][163,164], electrocatalysis [82][83][165,166], photocatalysis [84][85][167,168], and heterogeneous catalysis [77][81][82][83][86][23,164,165,166,169].
