Alcohol is one of the commonly used drugs. Ethanol, together with its derivatives and metabolites, exhibits diverse direct and indirect toxic effects, which are responsible for damage to many organs. This toxicity depends on many factors, such as dose, gender, associated comorbidities, or genetic predisposition.
- ethanol
- acetaldehyde
- metabolization
- oxidative stress
- toxicity
1. Introduction
Although the negative effects of excessive alcohol consumption are generally known in the human population (Figure 1), the drinking of alcoholic beverages is prevalent in society. According to WHO, alcohol abuse contributes to three million deaths per year globally and millions of people’s disabilities and poor health. It is alarming that alcohol abuse is the cause of up to 10% of all deaths in the age group 15–49 years [1]. Acute intoxication is often the limit for ending ongoing consumption. In addition to acute intoxications, which often require urgent care attention, especially in young people, the danger of alcohol consumption lies primarily in alcohol addiction, leading to chronic abuse and organ damage. Affected organs/systems include the liver [2], the cardiovascular system [3], the endocrine system [4], the gastrointestinal tract [5], the neural system [6][7], or the metabolism of basic nutrients [8]. Although the mentioned organs/systems perform different functions, the distribution and metabolization of ethanol in organs/systems are variable. At the same time, there are also interrelationships in ethanol-related tissue damage, e.g., gastrointestinal or hepatic impairment may be associated with dysregulation of the immune system and vice versa [9]. There is also a relationship between chronic alcohol abuse and many types of cancer [10].
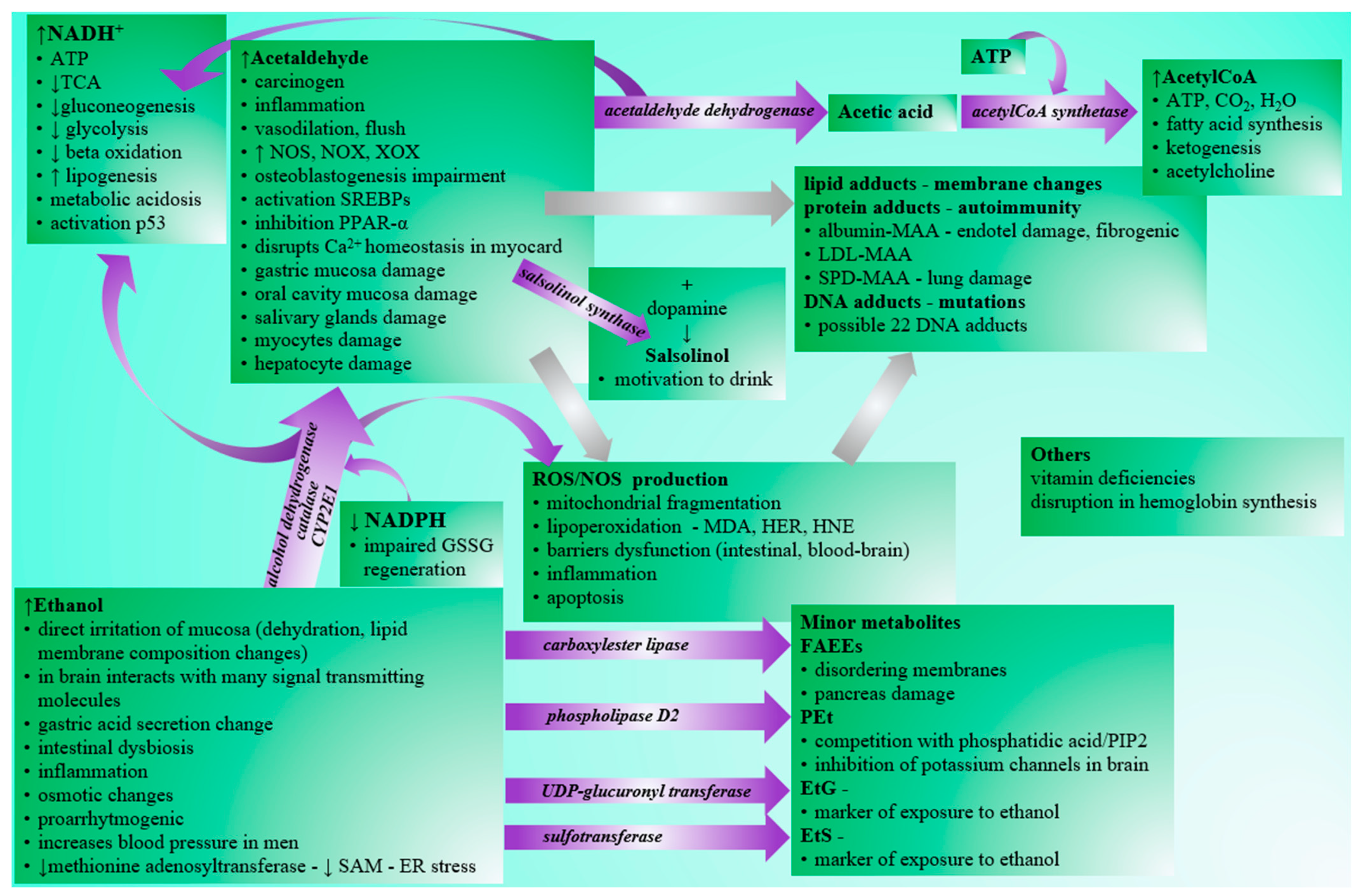
2. Alcohol Intake
The most common route of alcohol ingestion is oral. Ethanol uptake by inhalation or other routes is less common. Under some circumstances, ethanol can be created by one’s own body. The bacteria’s natural fermentation of saccharides in the gastrointestinal tract may lead to the production of trace amounts of endogenous ethanol. Dysmicrobia caused by Candida albicans or Saccharomyces species may promote ethanol production in the gut, as they produce acetaldehyde as the end product of anaerobic metabolism of pyruvate via the action of pyruvate decarboxylase. Accumulation of the acetaldehyde produced may lead to the synthesis of endogenous ethanol by a reversible reaction via bacterial alcohol dehydrogenase. This autogeneration of ethanol is not satisfactory to provide significant concentrations in peripheral venous blood from a forensic point of view [11]. However, nowadays, studies also confirm the effect of ethanol autoproduction on several pathological conditions, such as the increased intestinal permeability associated with liver steatosis [12] and non-alcoholic fatty liver disease [13][14]. In addition to the previously mentioned bacteria, also Gram-negative Klebsiella pneumoniae [15], Escherichia coli [16], and obligate anaerobes from Clostridia class [14] may colonize the gut of endogenous ethanol producers with possible pathological consequences. The production of endogenous ethanol and its metabolite acetaldehyde shows seasonal variation, and its ratio is important in body balance control during adaptation to cold [17].
Orally ingested alcohol is rapidly absorbed. The absorption of ethanol from the gut is dependent on several factors. These may be the time of the day, hydration state, the dosage and concentration of ingested ethanol, or the fed/fasting state of the drinking individual [18]. Ethanol passes freely through the biological membranes according to the concentration gradient and quickly reaches equilibrium with the plasma content. First-pass metabolization is present by the stomach and liver [19]. Ethanol is transported freely in plasma, without plasma protein transporter required. The transfer into the tissues can be affected by the relative water content of the tissues or cells, so that the distribution of ethanol may vary between tissues [20]. Therefore, the volume of ethanol distribution is lower during dehydration and in women or the elderly due to lower muscle mass [18]. A minor fraction of ingested ethanol undergoes biotransformation prior to urinary excretion via glucuronidation to form ethyl glucuronide EtG (0.6–1.5%) or via sulfation to form ethyl sulfate EtS (0.1%) [19][21]. Both glucuronidation and the sulfation of ethanol are possible in the lungs and liver, and the median time to appearance of ethyl glucuronide and ethyl sulfate is approximately 20 h [22]. In trace amounts, ethyl glucuronide accumulates in the hair and is currently one of the most reliable biomarkers of long-term alcohol exposure [23][24]. Most ethanol is oxidized via several possible enzymatic pathways, and the liver is the major organ (but not the only one) for its metabolization.
3. Metabolization of Ethanol
There are three pathways of enzymatic oxidation of ethanol to ethanal in humans. These are catalyzed via alcohol dehydrogenase (ADH), catalase, or CYP2E1.
Information on the variable expression of alcohol dehydrogenase (ADH) points to an essential role of organs other than the liver in ethanol metabolism and possible toxic effects in non-liver tissues. Cells within the gastrointestinal tract expressing various forms of ADH are vital for the first-pass effect of ethanol [25]. Lungs are an important organ for the excretion of unchanged ethanol and the ethanol’s biotransformation and oxidation [26].
The structure of ADH and the reaction mechanism are currently thought to be well described and understood [27][28], but a new report on the requirement of ATP for the ADHA1 action is not yet included in textbooks [29]. In addition, inhibition of ADH activity may lead to increased ethanol metabolism via other pathways. An example is aspirin and salicylate, with the variable extent of inhibition of multiple forms of ADH isoenzymes, which may be competitive or non-competitive [30], so the concomitant use of ethanol with widely used drugs with this effect may aggravate the formation of other potentially harmful metabolic intermediates and byproducts.
Although catalase is reported to be involved in ethanol metabolization, some authors claim that the reaction is improbable to occur under in vivo conditions due to the lack of hydrogen peroxide necessary for the reaction [18]. Other authors highlight the significant role of catalase in the brain, in which this enzyme may be responsible for the conversion of 60% of ethanol to acetaldehyde. Oxidation by CYP2E1 also occurs in the brain, and the alcohol dehydrogenase action is minor, if any [31][32]. This claim is supported by the fact that the brain produces acetaldehyde from ethanol even under conditions with pyrazole-inhibited alcohol dehydrogenase [33]. Under conditions with high activity of aldehyde oxidase and xanthine oxidase, which are important producers of hydrogen peroxide, the oxidation of ethanol by the action of catalase in the liver also increases [34][35].
Most of the ethanal is enzymatically converted to acetylCoA in two steps (via aldehyde dehydrogenase and acetylCoA synthetase).
4. Toxicity of Ethanol
Various behavioral and metabolic effects occur after the ingestion of alcoholic beverages. Blood alcohol levels around 12 mM are associated with anxiolytic and euphoric effects. An increase in ethanol concentration is related to slowed response times, motor and cognitive impairment, and increased sedation. Above 50 mM, this level of sedation and respiratory depression can lead to coma or death [36]. Tolerance to acute manifestations may develop, and adapted humans may survive eight-fold higher concentrations compared to ethanol-naive persons [37]. Ethanol itself but also some compounds resulting from its metabolization, such as acetaldehyde, NADH, acetylCoA, fatty acids ethyl esters (FAEEs), and phosphatidyl ethanol (PEt), cause general manifestations related to toxicity.
Gastrointestinal tract epithelium is one of the fastest renewing tissues, a vital barrier, an immune organ, and ethanol’s first-pass metabolism site. Consequently, ethanol has pathological effects on intestinal function due to loss of barrier integrity, causing local and systemic inflammation and microbial dysbiosis [38].
Excessive alcohol intake induces morphological changes of the villus surface and crypt deformation similar to intestinal bowel diseases. Moreover, ethanol stimulates the proliferation of the small intestinal epithelial cells and intestinal stem cells in intestinal crypts via increased expression of cyclin D1, Ki67 which are proliferation markers and Wnt target genes essential in the self-renewal of small intestinal stem cells and the generation of Paneth cells located nearby the stem cells [39].
In the central nervous system, the region-specific metabolism of ethanol is present. For most cells, the dominant ethanol-oxidizing enzymes are catalase (60%, Km = 12 mM) and CYP2E1 (20%, Km 8–10 mM) [40]. The presence of alcohol dehydrogenase is limited on low expressed ADH1B and ADH1C in the cerebellum (Table 1). The mitochondrial isoform of ALDH2 oxidizes acetaldehyde to acetate [33]. Since ethanol processing in the central nervous system uses catalase or CYP2E1 in the first step, it is more associated with oxidative stress in the CNS than in other localities. In the brain, the toxic effects of ethanol and acetaldehyde described earlier predominate. Concentrations of acetaldehyde in the blood and brain do not correspond, as the blood–brain barrier is not permeable for acetaldehyde [41]. In astrocytes, acetaldehyde is a more potent toxin than ethanol, and both modulate the production of proinflammatory interleukin 6 and TNF-α [42]. The overproduction of NADH in the brain is lower than in other cells, mainly using ADH as an ethanol-oxidizing system. Acetate produced from acetaldehyde can be converted to neurotransmitter acetylcholine or used in the tricarboxylic cycle in the form of acetylCoA. AcetylCoA can compensate for energy deficit caused by ethanol-induced decreased glucose uptake. At the same time, glucose is also essential for the pentose phosphate pathway as an NADPH source. Inherited tolerance to the hypnotic effect of ethanol in rats is related to the better utilization of acetate for acetylCoA and acetylcholine synthesis [43]. In the central nervous system, there are also interesting co-addictions involving ethanol, for example, the co-addiction of ethanol and nicotine [40]. Minor metabolites of ethanol as PEt or salsolinol may become more important in understanding the ethanol-induced effects in the brain in the future.
| Class | ADH Subunit | Tissue Expression | Cell Compartment | Substrates |
|---|---|---|---|---|
| I | ADH1A active in fetal life low in adults |
MPES: liver LPES: duodenum, small intestine [44] |
|
|
| ADH1B major role in ethanol oxidation |
HPES: liver MPES: duodenum, small intestine LPES: cerebellum, oral mucosa, esophagus, stomach, rectum, gallbladder, kidney, urinary bladder, vagina, breast, heart muscle, appendix, tonsils [49] |
|
||
| ADH1C major role in ethanol oxidation |
HPES: liver MPES: stomach, duodenum, small intestine, rectum, gallbladder, kidney, urinary bladder, epididymis, seminal vesicle, breast, appendix LPES: cerebellum, basal ganglia, hippocampus, colon, testis, vagina, fallopian tube, heart muscle, skeletal muscle, adipose tissue, spleen, lymph node, tonsil, bone marrow [51] |
|
||
| II | ADH4 | HPES: liver MPES: duodenum LPES: small intestine [53] |
|
|
| III | ADH5 ineffective in ethanol oxidation |
HPES: liver, kidney, testis, epididymis, smooth muscle MPES: thyroid gland, adrenal gland, nasopharynx, bronchus, lung, oral mucosa, salivary gland, esophagus, stomach, duodenum, small intestine, colon, rectum, gallbladder, pancreas, urinary bladder, seminal vesicle, prostate, vagina, ovary, endometrium, cervix, heart muscle, skin, appendix LPES: cerebral cortex, hippocampus, parathyroid gland, fallopian tube, placenta, breast, tonsil [56] |
|
|
| IV | ADH6 contains steroid hormone receptor binding site [58] |
MPES: liver, gallbladder, duodenum, small intestine LPES: stomach, rectum, kidney [59] |
|
|
| V | ADH7 ineffective in ethanol oxidation |
HPES: nasopharynx, bronchus, oral mucosa, esophagus MPES: stomach, tonsil LPES: colon [61] |
|
|
References
- World Health Organization. Harmful Use of Alcohol. NMH Fact Sheet 2009. Available online: https://www.who.int/nmh/publications/fact_sheet_alcohol_en.pdf (accessed on 20 July 2021).
- Askgaard, G.; Kraglund, F.; Kann, A.E.; Vilstrup, H.; Jepsen, P. Epidemiology for alcohol-related liver disease. Ugeskr. Laeger 2021, 183, 14.
- Grubb, A.F.; Greene, S.J.; Fudim, M.; Dewald, T.; Mentz, R.J. Drugs of Abuse and Heart Failure. J. Card. Fail. 2021.
- Farokhnia, M.; Abshire, K.M.; Hammer, A.; Deschaine, S.L.; Saravanakumar, A.; Cobbina, E.; You, Z.-B.; Haass-Koffler, C.L.; Lee, M.R.; Akhlaghi, F.; et al. Neuroendocrine Response to Exogenous Ghrelin Administration, Combined With Alcohol, in Heavy-Drinking Individuals: Findings From a Randomized, Double-Blind, Placebo-Controlled Human Laboratory Study. Int. J. Neuropsychopharmacol. 2021, 24, 464–476.
- Kubák, M.; Gavurová, B.; Kulhánek, A. Spatial analysis of alcohol-related mortality in Slovakia. Central Eur. J. Public Health 2019, 27, S48–S54.
- Şenadım, S.; Baslo, S.A.; Uygun, E.; Erdoğan, M.; Balçik, Z.E.; Tekin, B.; Ataklı, D. The strategies for coping with stress of epilepsy patients. Neurol. Sci. 2021, 1–6.
- Lv, X.; Hong, Q.; Lin, X.; Chen, W.; Tian, Y. Osmotic Demyelination Syndrome: Clinical, Neuroimaging Characteristics, and Outcomes in a Series of 18 Cases. BioMed Res. Int. 2021, 2021, 1–9.
- Varghese, D.S.; Ali, B.R. Pathological Crosstalk Between Oxidized LDL and ER Stress in Human Diseases: A Comprehensive Review. Front. Cell Dev. Biol. 2021, 9, 1276.
- Santiesteban-Lores, L.E.; Carneiro, M.C.; Isaac, L.; Bavia, L. Complement System in Alcohol-Associated Liver Disease. Immunol. Lett. 2021, 236, 37–50.
- Calvert, C.M.; Toomey, T.; Jones-Webb, R. Are people aware of the link between alcohol and different types of Cancer? BMC Public Health 2021, 21, 1–10.
- Logan, B.K.; Jones, A.W. Endogenous Ethanol ‘Auto-Brewery Syndrome’ as a Drunk-Driving Defence Challenge. Med. Sci. Law 2000, 40, 206–215.
- Ghetti, F.D.F.; Oliveira, D.G.; De Oliveira, J.M.; de Castro Ferreira, L.E.V.V.; Cesar, D.E.; Moreira, A.P.B. Influence of gut microbiota on the development and progression of nonalcoholic steatohepatitis. Eur. J. Nutr. 2017, 57, 861–876.
- Bibbò, S.; Ianiro, G.; Dore, M.P.; Simonelli, C.; Newton, E.E.; Cammarota, G. Gut Microbiota as a Driver of Inflammation in Nonalcoholic Fatty Liver Disease. Mediat. Inflamm. 2018, 2018, 1–7.
- Ruuskanen, M.O.; Åberg, F.; Männistö, V.; Havulinna, A.S.; Méric, G.; Liu, Y.; Loomba, R.; Vázquez-Baeza, Y.; Tripathi, A.; Valsta, L.M.; et al. Links between gut microbiome composition and fatty liver disease in a large population sample. Gut Microbes 2021, 13, 1–22.
- Yuan, J.; Chen, C.; Cui, J.; Lu, J.; Yan, C.; Wei, X.; Zhao, X.; Li, N.; Li, S.; Xue, G.; et al. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab. 2019, 30, 675–688.e7.
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2012, 57, 601–609.
- Kolosova, O.N.; Kershengolts, B.M.; Solomonov, N.G. Evolutionary Changes in the Content of Dehydrogenase System Metabolites as a Mechanism of Adaptation to Cold in Higher Vertebrates. Dokl. Biol. Sci. 2018, 482, 170–173.
- Norberg, A.; Jones, A.W.; Hahn, R.G.; Gabrielsson, J.L. Role of Variability in Explaining Ethanol Pharmacokinetics: Research and Forensic Applications. Clin. Pharmacokinet. 2003, 42, 1–31.
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol Res. Health 2006, 29, 245.
- Cederbaum, A.I. Alcohol Metabolism. Clin. Liver Dis. 2012, 16, 667–685.
- Woźniak, M.K.; Banaszkiewicz, L.; Aszyk, J.; Wiergowski, M.; Jańczewska, I.; Wierzba, J.; Kot-Wasik, A.; Biziuk, M. Development and validation of a method for the simultaneous analysis of fatty acid ethyl esters, ethyl sulfate and ethyl glucuronide in neonatal meconium: Application in two cases of alcohol consumption during pregnancy. Anal. Bioanal. Chem. 2021, 413, 3093–3105.
- Wurst, F.M.; Dresen, S.; Allen, J.P.; Wiesbeck, G.; Graf, M.; Weinmann, W. Ethyl sulphate: A direct ethanol metabolite reflecting recent alcohol consumption. Addiction 2006, 101, 204–211.
- Müller, A.; Iwersen-Bergmann, S. Determination of ethyl glucuronide in human hair samples: Decontamination vs extraction. Drug Test. Anal. 2020, 12, 948–956.
- Mueller, A.; Jungen, H.; Iwersen-Bergmann, S.; Raduenz, L.; Lezius, S.; Andresen-Streichert, H. Determination of ethyl glucuronide in human hair samples: A multivariate analysis of the impact of extraction conditions on quantitative results. Forensic Sci. Int. 2016, 271, 43–48.
- Farrés, J.; Moreno, A.; Crosas, B.; Peralba, J.M.; Allali-Hassani, A.; Hjelmqvist, L.; Jörnvall, H.; Parés, X. Alcohol Dehydrogenase of Class IV (Σσ-ADH) from Human Stomach: cDNA Sequence and Structure/Function Relationships. JBIC J. Biol. Inorg. Chem. 1994, 224, 549–557.
- Kaphalia, L.; Calhoun, W.J. Alcoholic lung injury: Metabolic, biochemical and immunological aspects. Toxicol. Lett. 2013, 222, 171–179.
- Niederhut, M.S.; Gibbons, B.J.; Perez-Miller, S.; Hurley, T.D. Three-dimensional structures of the three human class I alcohol dehydrogenases. Protein Sci. 2001, 10, 697–706.
- Brandt, E.G.; Hellgren, M.; Brinck, T.; Bergman, T.; Edholm, O. Molecular dynamics study of zinc binding to cysteines in a peptide mimic of the alcohol dehydrogenase structural zinc site. Phys. Chem. Chem. Phys. 2009, 11, 975–983.
- Khmelinskii, I.; Makarov, V.I. Reaction coupling in ADH1A alcohol dehydrogenase enzyme by exciplex formation with adenosine diphosphate moderated by low-energy electronic excited states. Phys. Rev. E 2021, 103, 052405.
- Lee, S.-L.; Lee, Y.-P.; Wu, M.-L.; Chi, Y.-C.; Liu, C.-M.; Lai, C.-L.; Yin, S.-J. Inhibition of human alcohol and aldehyde dehydrogenases by aspirin and salicylate: Assessment of the effects on first-pass metabolism of ethanol. Biochem. Pharmacol. 2015, 95, 71–79.
- Zimatkin, S.M.; Pronko, S.P.; Vasiliou, V.; Gonzalez, F.J.; Deitrich, R.A. Enzymatic Mechanisms of Ethanol Oxidation in the Brain. Alcohol. Clin. Exp. Res. 2006, 30, 1500–1505.
- Zimatkin, S.M.; Buben, A.L. Ethanol oxidation in the living brain. Alcohol Alcohol. 2007, 42, 529–532.
- Deitrich, R.; Zimatkin, S.; Pronko, S. Oxidation of Ethanol in the Brain and Its Consequences. Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2006, 29, 266–273.
- Shaw, S.; Jayatilleke, E. The role of aldehyde oxidase in ethanol-induced hepatic lipid peroxidation in the rat. Biochem. J. 1990, 268, 579–583.
- Villalobos-García, D.; Hernández-Muñoz, R. Catalase increases ethanol oxidation through the purine catabolism in rat liver. Biochem. Pharmacol. 2017, 137, 107–112.
- Alifimoff, J.K.; Firestone, L.L.; Miller, K.W. Anaesthetic potencies of primary alkanols: Implications for the molecular dimensions of the anaesthetic site. Br. J. Pharmacol. 1989, 96, 9–16.
- Johnson, R. Survival after a serum ethanol concentration of 1 1/2%. Lancet 1982, 320, 1394.
- Leclercq, S.; Matamoros, S.; Cani, P.D.; Neyrinck, A.; Jamar, F.; Stärkel, P.; Windey, K.; Tremaroli, V.; Bäckhed, F.; Verbeke, K.; et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. USA 2014, 111, E4485–E4493.
- Park, J.-H.; Jung, I.K.; Lee, Y.; Jin, S.; Yun, H.J.; Kim, B.W.; Kwon, H.J. Alcohol stimulates the proliferation of mouse small intestinal epithelial cells via Wnt signaling. Biochem. Biophys. Res. Commun. 2020, 534, 639–645.
- Heit, C.; Dong, H.; Chen, Y.; Thompson, D.C.; Deitrich, R.A.; Vasiliou, V.K. The Role of CYP2E1 in Alcohol Metabolism and Sensitivity in the Central Nervous System. Subcell. Biochem. 2013, 67, 235–247.
- Deitrich, R. Ethanol as a Prodrug: Brain Metabolism of Ethanol Mediates Its Reinforcing Effects—A Commentary. Alcohol. Clin. Exp. Res. 2011, 35, 581–583.
- Sarc, L.; Wraber, B.; Lipnik-Stangelj, M. Ethanol and acetaldehyde disturb TNF-alpha and IL-6 production in cultured astrocytes. Hum. Exp. Toxicol. 2010, 30, 1256–1265.
- Zimatkin, S.M.; Oganesian, N.A.; Kiselevski, Y.V.; Deitrich, R.A. Acetate-Dependent Mechanisms of Inborn Tolerance to Ethanol. Alcohol Alcohol. 2011, 46, 233–238.
- ADH1A Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000187758-ADH1A/tissue (accessed on 25 August 2021).
- ADH1A Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000187758-ADH1A/cell (accessed on 25 August 2021).
- ADH1A Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org//ENSG00000187758-ADH1A (accessed on 25 August 2021).
- ADH1B Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000196616-ADH1B (accessed on 25 August 2021).
- ADH1C Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000248144-ADH1C (accessed on 25 August 2021).
- ADH1B Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000196616-ADH1B/tissue (accessed on 25 August 2021).
- ADH1B Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000196616-ADH1B/cell (accessed on 25 August 2021).
- ADH1C Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000248144-ADH1C/tissue (accessed on 25 August 2021).
- ADH1C Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000248144-ADH1C/cell (accessed on 25 August 2021).
- ADH4 Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000198099-ADH4/tissue (accessed on 25 August 2021).
- ADH4 Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000198099-ADH4/cell (accessed on 25 August 2021).
- ADH4 Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000198099-ADH4 (accessed on 25 August 2021).
- ADH5 Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000197894-ADH5/tissue (accessed on 25 August 2021).
- ADH5 Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000197894-ADH5 (accessed on 25 August 2021).
- ADH6 Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000172955-ADH6 (accessed on 25 August 2021).
- ADH6 Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000172955-ADH6/tissue (accessed on 25 August 2021).
- Reactome|ADH6 [cytosol]. Available online: http://www.reactome.org/content/detail/R-HSA-71747 (accessed on 25 August 2021).
- ADH7 Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000196344-ADH7/tissue (accessed on 25 August 2021).
- ADH7 Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000196344-ADH7/cell (accessed on 25 August 2021).
- ADH7 Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000196344-ADH7 (accessed on 25 August 2021).
