All the examined works reported an increase in oil yield and extractability index through the use of high-power ultrasound(HPU), which was ascribed to reduced paste viscosity and cavitation-driven cell disruption. Olive oil legal quality was generally not affected; on the other hand, results regarding oil chemical composition were conflicting with some studies reporting an increase of phenols, tocopherols, and volatile compounds, while others underlined no significant effects to even slight reductions after HPU treatment. Regarding the acceptability of oils extracted through HPU processing, consumer perception is not negatively affected, as long as the marketer effectively delivers information about the positive effects of ultrasound on oil quality and sensory aspect.
- olive oil processing
- malaxation
- ultrasound
- innovative technology
1. Introduction
| Application | Food Matrix | Ultrasound Power | References | |||
|---|---|---|---|---|---|---|
| Cooking velocity | Mortadella | 301 W | [22] | |||
| Freezing speed up | Potatoes | 270 W | [23] | |||
| Fruit drying/dehydration | Bananas/Apples | 4000/4870 W/m | 2 | [26,27] | [26][27] | |
| Cutting | Cheese | 750 W | [28] | |||
| Filtration | Grape pomace extract | 400 W | [29] | |||
| Emulsification | Nanoemulsions | 450 W | [30] | |||
| Bioactives extraction | Pomegranate/Tea | 400/600 W | [31, | [31 | 32] | ][32] |
| Microbial inactivation | Apple juice | 600 W | [33] | |||
| Enzyme inactivation/modulation | Carrots/Lipases | 400 W/2–21 W/cm | 2 | [24,25] | [24][25] |
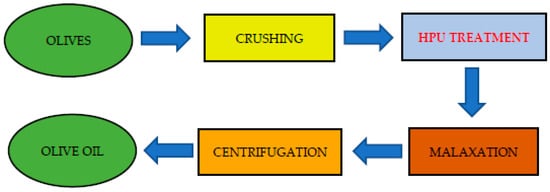
2. Effect of HPU on Olive Oil Yield and Extractability
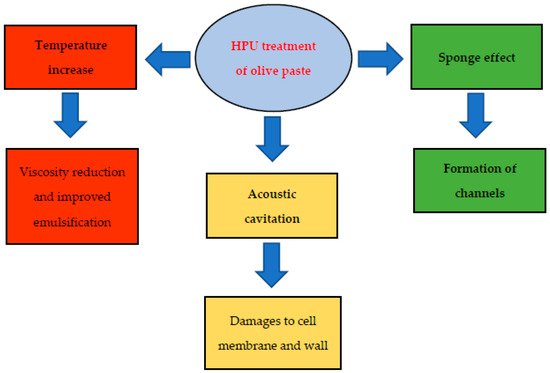
3. Effect of HPU on Olive Oil Quality and Chemical Composition
3.1. Legal Quality Indices
3.2. Total Phenolic Content
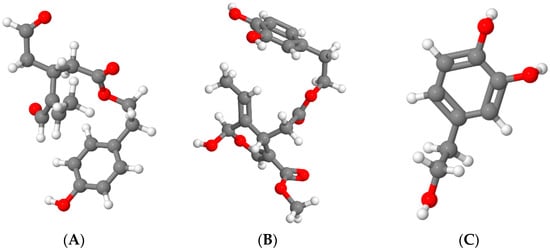
3.3. Lipid Stability and Fatty Acid Composition
3.4. Volatile Compounds and Sensory Properties
3.5. Other Physico-Chemical and Chemical Characteristics
References
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The secrets of the mediterranean diet. Does olive oil matter? Nutrients 2019, 11, 2941.
- Gerber, M.; Hoffman, R. The Mediterranean diet: Health, science and society. Br. J. Nutr. 2015, 113, S4–S10.
- Vilarnau, C.; Stracker, D.M.; Funtikov, A.; da Silva, R.; Estruch, R.; Bach-Faig, A. Worldwide adherence to Mediterranean Diet between 1960 and 2011. Eur. J. Clin. Nutr. 2019, 72, 83–91.
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, its Components, and Cardiovascular Disease. Am. J. Med. 2015, 128, 229–238.
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S.
- Gavahian, M.; Mousavi Khaneghah, A.; Lorenzo, J.M.; Munekata, P.E.S.; Garcia-Mantrana, I.; Collado, M.C.; Meléndez-Martínez, A.J.; Barba, F.J. Health benefits of olive oil and its components: Impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases. Trends Food Sci. Technol. 2019, 88, 220–227.
- George, E.S.; Marshall, S.; Mayr, H.L.; Trakman, G.L.; Tatucu-Babet, O.A.; Lassemillante, A.C.M.; Bramley, A.; Reddy, A.J.; Forsyth, A.; Tierney, A.C.; et al. The effect of high-polyphenol extra virgin olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 2772–2795.
- Loffredo, L.; Perri, L.; Nocella, C.; Violi, F. Antioxidant and antiplatelet activity by polyphenol-rich nutrients: Focus on extra virgin olive oil and cocoa. Br. J. Clin. Pharmacol. 2017, 83, 96–102.
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776.
- de Souza, P.A.L.; Marcadenti, A.; Portal, V.L. Effects of olive oil phenolic compounds on inflammation in the prevention and treatment of coronary artery disease. Nutrients 2017, 9, 1087.
- Luque-Sierra, A.; Alvarez-Amor, L.; Kleemann, R.; Martín, F.; Varela, L.M. Extra-Virgin Olive Oil with Natural Phenolic Content Exerts an Anti-Inflammatory Effect in Adipose Tissue and Attenuates the Severity of Atherosclerotic Lesions in Ldlr−/−.Leiden Mice. Mol. Nutr. Food Res. 2018, 62, 1800295.
- Violi, F.; Loffredo, L.; Pignatelli, P.; Angelico, F.; Bartimoccia, S.; Nocella, C.; Cangemi, R.; Petruccioli, A.; Monticolo, R.; Pastori, D.; et al. Extra virgin olive oil use is associated with improved post-prandial blood glucose and LDL cholesterol in healthy subjects. Nutr. Diabetes 2015, 5, e172-7.
- Rubio-Senent, F.; de Roos, B.; Duthie, G.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Inhibitory and synergistic effects of natural olive phenols on human platelet aggregation and lipid peroxidation of microsomes from vitamin E-deficient rats. Eur. J. Nutr. 2015, 54, 1287–1295.
- di Francesco, A.; Falconi, A.; di Germanio, C.; Micioni Di Bonaventura, M.V.; Costa, A.; Caramuta, S.; Del Carlo, M.; Compagnone, D.; Dainese, E.; Cifani, C.; et al. Extravirgin olive oil up-regulates CB1 tumor suppressor gene in human colon cancer cells and in rat colon via epigenetic mechanisms. J. Nutr. Biochem. 2015, 26, 250–258.
- Kalogianni, E.P.; Georgiou, D.; Hasanov, J.H. Olive Oil Processing: Current Knowledge, Literature Gaps, and Future Perspectives. JAOCS J. Am. Oil Chem. Soc. 2019, 96, 481–507.
- Bejaoui, M.A.; Sánchez-Ortiz, A.; Sánchez, S.; Jiménez, A.; Beltrán, G. The high power ultrasound frequency: Effect on the virgin olive oil yield and quality. J. Food Eng. 2017, 207, 10–17.
- Clodoveo, M.L.; Hachicha Hbaieb, R. Beyond the traditional virgin olive oil extraction systems: Searching innovative and sustainable plant engineering solutions. Food Res. Int. 2013, 54, 1926–1933.
- Kalua, C.M.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D. Changes in volatile and phenolic compounds with malaxation time and temperature during virgin olive oil production. J. Agric. Food Chem. 2006, 54, 7641–7651.
- Caponio, F.; Leone, A.; Squeo, G.; Tamborrino, A.; Summo, C. Innovative technologies in virgin olive oil extraction process: Influence on volatile compounds and organoleptic characteristics. J. Sci. Food Agric. 2019, 99, 5594–5600.
- Leone, A.; Tamborrino, A.; Romaniello, R.; Zagaria, R.; Sabella, E. Specification and implementation of a continuous microwave-assisted system for paste malaxation in an olive oil extraction plant. Biosyst. Eng. 2014, 125, 24–35.
- Veneziani, G.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Sordini, B.; Lorefice, A.; Daidone, L.; Pagano, M.; Tomasone, R.; Servili, M. Extra-Virgin Olive Oil Extracted Using Pulsed Electric Field Technology: Cultivar Impact on Oil Yield and Quality. Front. Nutr. 2019, 6, 1–8.
- da Silva, J.S.; Voss, M.; de Menezes, C.R.; Barin, J.S.; Wagner, R.; Campagnol, P.C.B.; Cichoski, A.J. Is it possible to reduce the cooking time of mortadellas using ultrasound without affecting their oxidative and microbiological quality? Meat Sci. 2020, 159, 107947.
- Zhu, Z.; Zhang, P.; Sun, D.W. Effects of multi-frequency ultrasound on freezing rates and quality attributes of potatoes. Ultrason. Sonochem. 2020, 60, 104733.
- Gamboa-Santos, J.; Montilla, A.; Soria, A.C.; Villamiel, M. Effects of conventional and ultrasound blanching on enzyme inactivation and carbohydrate content of carrots. Eur. Food Res. Technol. 2012, 234, 1071–1079.
- Nadar, S.S.; Rathod, V.K. Encapsulation of lipase within metal-organic framework (MOF) with enhanced activity intensified under ultrasound. Enzyme Microb. Technol. 2018, 108, 11–20.
- Fernandes, F.A.N.; Rodrigues, S. Ultrasound as pre-treatment for drying of fruits: Dehydration of banana. J. Food Eng. 2007, 82, 261–267.
- Nowacka, M.; Wiktor, A.; Śledź, M.; Jurek, N.; Witrowa-Rajchert, D. Drying of ultrasound pretreated apple and its selected physical properties. J. Food Eng. 2012, 113, 427–433.
- Yildiz, G.; Rababah, T.M.; Feng, H. Ultrasound-assisted cutting of cheddar, mozzarella and Swiss cheeses—Effects on quality attributes during storage. Innov. Food Sci. Emerg. Technol. 2016, 37, 1–9.
- Liu, D.; Vorobiev, E.; Savoire, R.; Lanoisellé, J.L. Comparative study of ultrasound-assisted and conventional stirred dead-end microfiltration of grape pomace extracts. Ultrason. Sonochem. 2013, 20, 708–714.
- Shao, Y.; Wu, C.; Wu, T.; Li, Y.; Chen, S.; Yuan, C.; Hu, Y. Eugenol-chitosan nanoemulsions by ultrasound-mediated emulsification: Formulation, characterization and antimicrobial activity. Carbohydr. Polym. 2018, 193, 144–152.
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT 2019, 101, 342–350.
- Horžić, D.; Jambrak, A.R.; Belščak-Cvitanović, A.; Komes, D.; Lelas, V. Comparison of Conventional and Ultrasound Assisted Extraction Techniques of Yellow Tea and Bioactive Composition of Obtained Extracts. Food Bioprocess Technol. 2012, 5, 2858–2870.
- Gabriel, A.A. Microbial inactivation in cloudy apple juice by multi-frequency Dynashock power ultrasound. Ultrason. Sonochem. 2012, 19, 346–351.
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902.
- Jadhav, A.J.; Holkar, C.R.; Goswami, A.D.; Pandit, A.B.; Pinjari, D.V. Acoustic Cavitation as a Novel Approach for Extraction of Oil from Waste Date Seeds. ACS Sustain. Chem. Eng. 2016, 4, 4256–4263.
- Bermúdez-Aguirre, D.; Mobbs, T.; Barbosa-Cánovas, G.V. Ultrasound applications in food processing. In Ultrasound Technologies for Food and Bioprocessing; Feng, H., Barbosa-Cánovas, G.V., Weiss, J., Eds.; Springer: New York, NY, USA, 2011; pp. 65–105.
- de São José, J.F.B.; Andrade, N.J.; de Ramos, A.M.; Vanetti, M.C.D.; Stringheta, P.C.; Chaves, J.B.P. Decontamination by ultrasound application in fresh fruits and vegetables. Food Control 2014, 45, 36–50.
- Amirante, R.; Distaso, E.; Tamburrano, P.; Paduano, A.; Pettinicchio, D.; Clodoveo, M.L. Acoustic cavitation by means ultrasounds in the extra virgin olive oil extraction process. Energy Procedia 2017, 126, 82–90.
- Bejaoui, M.A.; Beltran, G.; Aguilera, M.P.; Jimenez, A. Continuous conditioning of olive paste by high power ultrasounds: Response surface methodology to predict temperature and its effect on oil yield and virgin olive oil characteristics. LWT Food Sci. Technol. 2016, 69, 175–184.
- Iqdiam, B.M.; Mostafa, H.; Goodrich-Schneider, R.; Baker, G.L.; Welt, B.; Marshall, M.R. High Power Ultrasound: Impact on Olive Paste Temperature, Malaxation Time, Extraction Efficiency, and Characteristics of Extra Virgin Olive Oil. Food Bioprocess Technol. 2018, 11, 634–644.
- Iqdiam, B.M.; Abuagela, M.O.; Marshall, S.M.; Yagiz, Y.; Goodrich-Schneider, R.; Baker, G.L.; Welt, B.A.; Marshall, M.R. Combining high power ultrasound pre-treatment with malaxation oxygen control to improve quantity and quality of extra virgin olive oil. J. Food Eng. 2019, 244, 1–10.
- Juliano, P.; Bainczyk, F.; Swiergon, P.; Supriyatna, M.I.M.; Guillaume, C.; Ravetti, L.; Canamasas, P.; Cravotto, G.; Xu, X.Q. Extraction of olive oil assisted by high-frequency ultrasound standing waves. Ultrason. Sonochem. 2017, 38, 104–114.
- Rigane, G.; Yahyaoui, A.; Acar, A.; Mnif, S.; Salem, R.B.; Arslan, D. Change in some quality parameters and oxidative stability of olive oils with regard to ultrasound pretreatment, depitting and water addition. Biotechnol. Rep. 2020, 26, 4–9.
- Servili, M.; Veneziani, G.; Taticchi, A.; Romaniello, R.; Tamborrino, A.; Leone, A. Low-frequency, high-power ultrasound treatment at different pressures for olive paste: Effects on olive oil yield and quality. Ultrason. Sonochem. 2019, 59, 104747.
- Cárcel, J.A.; Benedito, J.; Rosselló, C.; Mulet, A. Influence of ultrasound intensity on mass transfer in apple immersed in a sucrose solution. J. Food Eng. 2007, 78, 472–479.
- Kentish, S.; Ashokkumar, M. The physical and chemical effects of ultrasound. In Ultrasound Technologies for Food and Bioprocessing; Feng, H., Barbosa-Cánovas, G.V., Weiss, J., Eds.; Springer: New York, NY, USA, 2011; pp. 1–12.
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872.
- Tamborrino, A.; Romaniello, R.; Caponio, F.; Squeo, G.; Leone, A. Combined industrial olive oil extraction plant using ultrasounds, microwave, and heat exchange: Impact on olive oil quality and yield. J. Food Eng. 2019, 245, 124–130.
- IOC (INTERNATIONAL OLIVE COUNCIL). Trade standard applying to olive oils and olive pomace oils. Int. Olive Counc. 2019, 15, 1–17.
- European Commission Implementing Regulation (EU) no 299/2013 of March 2013 Amending Regulation (EEC) no. 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. Off. J. Eur. Union 2013, 90, 52–70. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:090:0052:0070:EN:PDF (accessed on 27 October 2021).
- Taticchi, A.; Selvaggini, R.; Esposto, S.; Sordini, B.; Veneziani, G.; Servili, M. Physicochemical characterization of virgin olive oil obtained using an ultrasound-assisted extraction at an industrial scale: Influence of olive maturity index and malaxation time. Food Chem. 2019, 289, 7–15.
- Clodoveo, M.L.; Durante, V.; La Notte, D. Working towards the development of innovative ultrasound equipment for the extraction of virgin olive oil. Ultrason. Sonochem. 2013, 20, 1261–1270.
- Artajo, L.S.; Romero, M.P.; Morelló, J.R.; Motilva, M.J. Enrichment of refined olive oil with phenolic compounds: Evaluation of their antioxidant activity and their effect on the bitter index. J. Agric. Food Chem. 2006, 54, 6079–6088.
- Dierkes, G.; Krieger, S.; Dück, R.; Bongartz, A.; Schmitz, O.J.; Hayen, H. High-performance liquid chromatography-mass spectrometry profiling of phenolic compounds for evaluation of olive oil bitterness and pungency. J. Agric. Food Chem. 2012, 60, 7597–7606.
- Clodoveo, M.L.; Paduano, A.; Di Palmo, T.; Crupi, P.; Moramarco, V.; Distaso, E.; Tamburrano, P.; Amirante, R.; Sacchi, R.; Corbo, F.; et al. Engineering design and prototype development of a full scale ultrasound system for virgin olive oil by means of numerical and experimental analysis. Ultrason. Sonochem. 2017, 37, 169–181.
- Del Coco, L.; Girelli, C.R.; Angilè, F.; Mascio, I.; Montemurro, C.; Distaso, E.; Tamburrano, P.; Chiurlia, S.; Clodoveo, M.L.; Corbo, F.; et al. NMR-based metabolomic study of Apulian Coratina extra virgin olive oil extracted with a combined ultrasound and thermal conditioning process in an industrial setting. Food Chem. 2021, 345, 128778.
- Bejaoui, M.A.; Sánchez-Ortiz, A.; Aguilera, M.P.; Ruiz-Moreno, M.J.; Sánchez, S.; Jiménez, A.; Beltrán, G. High power ultrasound frequency for olive paste conditioning: Effect on the virgin olive oil bioactive compounds and sensorial characteristics. Innov. Food Sci. Emerg. Technol. 2018, 47, 136–145.
- Yahyaoui, A.; Rigane, G.; Mnif, S.; Salem, R.B.; Acar, A.; Arslan, D. Ultrasound Technology Parameters: Effects on Phenolics in Olive Paste and Oil in Relation to Enzymatic Activity. Eur. J. Lipid Sci. Technol. 2019, 121, 1800295.
- Almeida, B.; Valli, E.; Bendini, A.; Gallina Toschi, T. Semi-industrial ultrasound-assisted virgin olive oil extraction: Impact on quality. Eur. J. Lipid Sci. Technol. 2017, 119, 1600230.
- Jmol: An Open-Source Java Viewer for Chemical Structures in 3D. Available online: http://www.jmol.org/ (accessed on 22 October 2021).
- Chanioti, S.; Tzia, C. Optimization of ultrasound-assisted extraction of oil from olive pomace using response surface technology: Oil recovery, unsaponifiable matter, total phenol content and antioxidant activity. LWT Food Sci. Technol. 2017, 79, 178–189.
- Lester, G.E.; Lewers, K.S.; Medina, M.B.; Saftner, R.A. Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin-Ciocalteu: Assay interference by ascorbic acid. J. Food Compos. Anal. 2012, 27, 102–107.
- Gila, A.; Sánchez-Ortiz, A.; Jiménez, A.; Beltrán, G. The ultrasound application does not affect to the thermal properties and chemical composition of virgin olive oils. Ultrason. Sonochem. 2021, 70, 105320.
- Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. Antioxidant capacity of individual and combined virgin olive oil minor compounds evaluated at mild temperature (25 and 40 C) as compared to accelerated and antiradical assays. Food Chem. 2014, 150, 374–381.
- Gardner, H.W. Oxygen radical chemistry of polyunsaturated fatty acids. Free Radic. Biol. Med. 1989, 7, 65–86.
- Chemat, F.; Grondin, I.; Costes, P.; Moutoussamy, L.; Sing, A.S.C.; Smadja, J. High power ultrasound effects on lipid oxidation of refined sunflower oil. Ultrason. Sonochem. 2004, 11, 281–285.
- Beltrán, G.; Del Rio, C.; Sánchez, S.; Martínez, L. Influence of harvest date and crop yield on the fatty acid composition of virgin olive oils from cv. Picual. J. Agric. Food Chem. 2004, 52, 3434–3440.
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286.
- Yao, Y. Enhancement of mass transfer by ultrasound: Application to adsorbent regeneration and food drying/dehydration. Ultrason. Sonochem. 2016, 31, 512–531.
