Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Carmen Teodosiu and Version 2 by Rita Xu.
Municipal wastewater treatment plants (MWWTPs) face great challenges in optimizing technologies and ensuring environmental sustainability, in direct correlation with the increased pollution with emerging and priority compounds, wastewater quality discharged and climate changes challenges. The recent developments on the synthesis and characterization of composites based on polyelectrolytes, are discussed, and a correlation of their actual structure and properties with the adsorption mechanisms and removal efficiencies of various pollutants in aqueous media (priority and emerging pollutants or other model pollutants) are presented.
- advanced wastewater treatment
- polyelectrolytes
- composites
- sorption
- heavy metals
- organic pollutants
1. Introduction
Municipal wastewater treatment plants (MWWTPs) face great challenges in optimizing technologies to avoid ecological and human health problems and to ensure environmental sustainability, in direct correlation with the increased pollution due to economic and social growth, wastewater quality discharged into surface waters and climate changes. Industrial activities are responsible for the discharge of effluents with a wide range of inorganic and organic compounds that belong to the priority (PPs) and emerging pollutants (EPs) classes, pharmaceuticals and personal care products, pesticides, heavy metals, detergents, flame-retardants being only few examples of such pollutants. Through their presence, eco-toxicological and human health effects, bio-accumulative and degradation characteristics may influence aquatic biota and the performances and costs of water and wastewater treatment technologies [1]. Moreover, considering the new Circular Economy Action Plan and EU Green Deal there is a huge pressure nowadays on the regional water operators (running water and wastewater treatment plants) to decrease their operational costs and associated environmental impacts and carbon footprints (especially due to energy consumption), while introducing more viable alternatives for wastewater recycling for industries, agriculture/irrigation, aquacultures, trying to recover materials and energy from wastewater sludge [2][3][2,3].
MWWTPs collecting wastewater from combined sewers usually remove solids of different sizes, biodegradable organic and inorganic compounds based on conventional processes such as the following: mechanical (bar-screens, grit removal and sedimentation), biological (suspended or attached growth) and tertiary (nitrogen and phosphorous removal) treatment. The implementation of wastewater reuse in agriculture or industry requires the elimination of targeted priority and emerging organic & inorganic pollutants, microorganisms (bacteria, viruses, parasites) by means of advanced wastewater treatment (AWWT) (such as membrane processes, advanced oxidation, adsorption, filtration, disinfection, etc.), that complete the conventional treatment as presented in Figure 1.
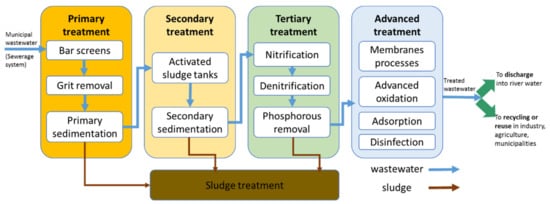
Figure 1. Municipal wastewater treatment outlines for various uses and pollutants removal.
Although, inorganic and organic pollutants classified as PPs or EPs are detected in wastewater at low concentrations (few micrograms/milligrams per liter), they are toxic, bio-accumulative, low biodegradable and very difficult to remove in terms of technological, energy and environmental efforts. In the European Union, both PPs and EPs are monitored in surface water [4][5][6][4,5,6], but at the moment, in Europe, only Switzerland enforces legal obligations to remove these compounds within MWWTPs [7]. The advanced wastewater treatment for EPs removal (for wastewater recycling and reuse) should consider at least the following criteria: (i) range of treated pollutants, treatment efficiency & removal mechanisms, (ii) environmental reduced impacts, (iii) simplicity of operation & maintenance, (iv) cost-effectiveness, (v) social acceptance [8].
In recent years, numerous classes of inorganic (metal oxides, zeolites, sand), organic (activated carbon, resins, covalent organic frameworks), or composite sorbents have been designed to sorb different classes of organic/inorganic pollutants [9][10][11][12][13][14][15][9,10,11,12,13,14,15]. Due to some disadvantages (high costs, low sorption capacities, low number of reusing cycles, non-degradable characteristics, secondary pollution) many types of these materials could be difficult to be used in practice at large scale. Recently, natural and synthetic polyelectrolytes were combined with some inorganic materials (SiO2, TiO2, graphene oxide, Fe3O4, clays, zeolites etc.) or other organic polymers (cellulose, wood, cyclodextrin, polystyrene (PS), etc.) to create new materials (composite type) as perfect candidates for sorption of toxic pollutants such as pharmaceuticals and heavy metal ions [16][17][16,17].
2. Composite Polyelectrolytes with Versatile Properties in Targeting Different Types of Pollutants Dissolved in Real/Simulated Aqueous Effluents
2.1. Why Composites Based on Polyelectrolytes?
Polyelectrolytes are charged/chargeable polymers whose repeated ionic/ionizable structural units are higher than 10–15 mol%. Based on their ability to partially/fully dissociate in aqueous environment, polyelectrolytes are usually classified in weak or strong and in anionic and cationic in dependence of charge type. These polymeric compounds have a great potential in water and wastewater treatment (such as coagulation-flocculation process) due to a particular characteristic: the high densities of functional groups bound to a flexible polymeric chain, allowing the intimate interaction at the atomic level with various types of pollutants.
Using polyelectrolyte characteristics, numerous types of composites can be fabricated by covalent bonding or non-covalent bonding (hydrogen bonding, π–π stacking, metal-ligand coordination, ionic, and hydrophobic interactions) between polyelectrolytes and different inorganic/organic partners (Table 1). Composite polyelectrolyte materials, which include mainly polymeric ionic chains, contain a large number and specific stimuli-responsive functional groups with controllable action (detection, immobilization, releasing) toward different classes of pollutants entities found in aqueous environment [18][19][18,19]. The main types of polyelectrolytes, polymers, and inorganics entities used in creation of composite sorbents, as well as the main tested pollutants, have been summarized in Table 1.
Table 1. Composite sorbents based on polyelectrolytes used for pollutants removal from aqueous media.
| Weak/Strong Polyelectrolytes | Organic/Inorganic Partner | Pollutant Targeted | References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chitosan (CS) | Poly(ethyleneimine), Poly(vinyl amine), Poly(vinyl alcohol), Poly( | N | , | N | -dimethylamino)ethyl methacrylate, Poly(methacrylic acid), Poly(sodium acrylate), carrageenan, carboxymethyl β-cyclodextrin, carboxyalkyl chitosan, Poly-hexamethylene guanidine, microcrystalline cellulose, sodium alginate, karaya gum, citric acid, itaconic acid, sodium dodecyl sulphate, Sodium lignosulfonate, graphene oxide (GO), Fe | 3 | O | 4 | , Fe, TiO | 2 | , Mesoporous silica structures (MCM-48), silicate rectorite, zeolite, succinic anhydride, maleic anhydride, itaconic acid, trans-aconitic acid, biochar | Congo Red (CR), Methyl Orange (MO), Methylene Blue (MB), Bromocresol Green (BCG), Reactive Black 5 (RB5), Acid blue-113, viruses, Fe(II), Fe(III), Cu(II), Ni(II), Co(II), Cr(III), Cr(VI), Zn(II) As(III), As(V), Cd(II), Pb(II), diclofenac, ciprofloxacin | [13][20][21][22][23][24][ | ,30 | ] | ,31,32 | [ | ,33 | 39] | ,34 | [40] | ,35 | [41] | ,36 | [ | ,37 | 42 | ,38 | ][43 | ,20 | [ | ,21,39 | ][44][45][46][47][48 | ,22 | 52 | ,23 | 25][26][27][28][29][30][31][32][33][34][35][36][37][38 | ,40 | ] | ,41,42 | [ | ,43 | 49] | ,44 | [50] | ,45 | [51]][ | ,24 | 53 | ,46 | ][ | ,25 | 54][ | ,26,47 | 55 | ,27,48 | ] | ,28,49,50,51,52,53,54 | [56][57] | [13,29,55,56,57] |
| Quaternized chitosan (QCS) | Chitosan, 3-chloro-2-hydroxypropyl trimethyl ammonium chloride, Fe | 3 | O | 4 | , GO | MO, CR, Cu(II), Fe(III), Cr(VI) | [20][58][59][60] | [20,58,59,60] | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sodium alginate (SA) | Activated carbon, bentonite, activated organo-bentonite, carboxy carbon nanotubes, pillared clay, Mauritanian clay, organo-illite/smectite clay, montmorillonite, nano-hydroxyapatite, carboxymethyl cellulose, microcrystalline cellulose, polyaniline, poly(acrylic acid) glutaraldehyde, Poly(hydroxybutyrate, biochar, CS, GO, Zr(IV), Fe | 3 | O | 4 | , MgAl-layered double hydroxide, SiO | 2 | , aluminum-based metal organic framework and chitosan | Nitrophenol, Pentachlorophenol, polychlorinated biphenyl, crystal violet (CV), MB, MO, As(V), Cu(II), Pb(II), Cd(II), Fe(III), F | - | , Cr(VI), bisphenol A | [14][33][56][59][61][62][63][64][65][66][67][68][69] | ,64 | [74 | ,65,66 | ] | ,67,68 | 70] | ,69 | [71] | ,70 | [72] | ,71 | [73] | ,72 | [[75 | ,73 | ][76 | ,74 | ] | ,75,76,77,78,79 | [81][82] | [14,33,56 | [77 | ,59 | ] | ,61 | [ | ,62 | 78][79][80 | ,63 | ] | ,80,81,82] | ||||||||||||||||||
| Carboxyalkyl chitosan (CCS) | CS, salecan, citric acid, Fe | 3 | O | 4 | , SiO | 2 | , Cu(II), Al(III), hexamethylenediisocyanate | As(III), As(V), Ni(II), Pb(II), ciprofloxacin | [52][83][84][85] | [52,83,84,85] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Modified Poly(Cyclodextrin) | 2,4-toluene diisocyanate, 1,6-hexamethylene diisocyanate, montmorillonite | 2,4-dinitrophenol, bisphenol A | [86][87] | [86,87] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poly(ethyleneimine) (PEI) | Chitosan, Epichlorohydrin (ECH), Poly(acrylic acid) (PAA), poly(vinyl amine), Poly(ethylene glycol) diglycidyl ether, diglycidyl ether of 1,4-butandiol, PS nanoparticles, montmorillonite, cellulose acetate, diatomaceous earth, bacteria, SiO | 2 | , CaCO | 3 | , Fe | 3 | O | 4 | Formaldehyde, CR, BCG, Rhodamine B, Hg(II), UO | 2 | (II), Cd(II), Zn(II), Cu(II), Ni(II), As(III), Mn(II), Cr(III), Cr(VI), Co(II), Fe(II), Pb(II), Zn(II) | [24][36][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103] | [24,36,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103] | |||||||||||||||||||||||||||||||||||||||||||||||
| Carboxymethyl cellulose (CMC) | SA, SiO | 2 | , | CR, MO, MB, BCG, Pb(II) | [24][79] | [24,79] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cationic cellulose | Cellulose nanocrystals, wood pulp | MB | [104] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poly(sodium 4-styrene sulfonate) (PSS) | Octacalcium phosphate, gelatin, pineapple leaf fiber, ZnO, Fe | 3 | O | 4 | Cu(II), Cd(II), tetracycline, CR, MB | [105][106][107][108] | [105,106,107,108] | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poly(vinyl amine) (PVAm) | CS, PEI | CR, BCG, Cu(II) | [54][109] | [54,109] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poly(acrylic acid) (PAA) | PEI, CS, Nano ferrous sulfide, SA | Cd(II), Cr(III), Cr(IV), Cr(VI), Cu(II) | [49][71][97] | [49,71,97] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carrageenan | chitosan, hybrid siliceous shells | CR, MB, metoprolol | [31][110] | [31,110] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Anionic polyacrylamide | Kaolinite, montmorillonite, xanthan gum, SiO | 2 | Cr(IV), Pb(II), oil | [111][112][113][114] | [111,112,113,114] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poly(allylamine hydrochloride) (PAH) | GO, diglycidyl ether of 1,4-butandiol | Cr(VI), Cu(II), Co(II), Zn(II), Ni(II) | [93][115][116] | [93,115,116] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poly(2-acrylamido-2-methylpropane sulfonic acid) (PAMPS) | Ti | 3 | C | 2 | -MXenes, methacrylic acid, 2-hydroxyethyl-methacrylate, gelatin, Fe | 3 | O | 4 | , CuO with chitosan, alumina | MB, Hg(II), Cu(II), Cd(II), Ni(II), Pb(II), Zn(II), doxycycline, ciprofloxacin | [106][117][118][119][120][121] | [106,117,118,119,120,121] | ||||||||||||||||||||||||||||||||||||||||||||||||
| Poly{[2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide- | co | -acrylic acid} (PDMAPS- | co | -AA) | Ti | 3 | C | 2 | -MXenes | MB | [117] | |||||||||||||||||||||||||||||||||||||||||||||||||
| Poly(diallyldimethylammonium chloride) (PDDA) | pineapple leaf fiber, ZnO | CR | [107] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Quaternized4-vinyl-pyridine- | co | -acrylamide (QVP- | co | -AAm) | Fe | 3 | O | 4 | CR | [122] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Humic acid (HA) | Fe | 3 | O | 4 | Cu(II) | [123] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poly{[2-(Methacryloyloxy)ethyl]trimethylammonium chloride} | N,N′ -Methylenebisacrylamide | Orange II, Remazol Brilliant Blue R | [124] |
The overview in Table 1 suggests the predominant use of heavy metals and dyes (HM&D) as models for inorganic and organic aqueous pollutants, respectively. From our testing experience, these pollutants present certain advantages as compared to more complex molecules, like emerging pollutants:
-
chemical structure—HM&D are usually present in solution in ionic form and have smaller molecular weights, allowing them to diffuse more easily through the sorbent’s pores and reach the active sites;
-
range of initial concentration during adsorption tests—HM&D are used up to hundreds of mg/L, while EPs usually are at most in the first few tens of mg/L;
-
analysis equipment—HM&D can be determined by using more affordable, less time consuming and potentially less sophisticated equipment (e.g., UV-Vis spectrophotometer, atomic absorption spectrometer).
Every sorption process of the active species implies physical (transport, flow, swelling, diffusion etc.) and chemical (interactions, immobilization) aspects. First of all, the pollutant molecules, dissolved in a certain medium, must be transported near to the active site of the composite sorbent. Then, based on physico-chemical interactions between sorbate and sorbent, the sorption process can take place. Therefore, the shape and size of each composite sorbent at macro/nanoscale will dictate the active specific surface available for subsequent chemical interactions. The swelling will influence the diffusion of sorbate through the dynamic pores and the composite particle size will dictate the potential applications at laboratory or industrial scale. For example, composites larger than 10 microns could be used in a column experimental set-up.
Thus, in this study all composites based on polyelectrolytes have been divided according to their macroscopic shape: (1) Beads—high porous materials with high accessibility to the functional moieties; (2) Core-shell, “hard-soft” composites with high surface area; (3) Gels (hydrogels, cryogel monoliths, sponges), stimuli-responsive systems with high surface area; (4) Nanofibers, presenting good mechanical properties with high specific area and (5) Membranes, with sorption and separation in one step (Figure 23).
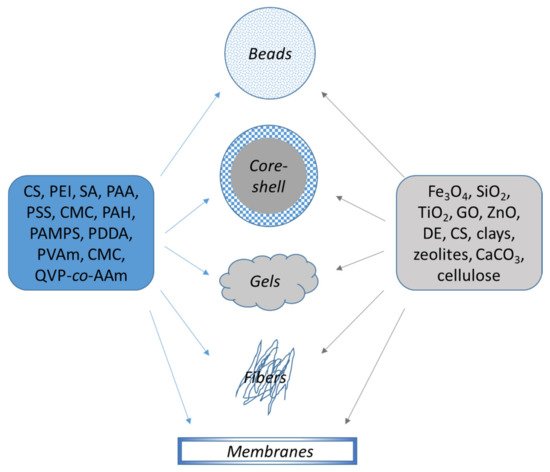

Figure 23. Organic/inorganic composite sorbents based on polyelectrolytes.
The use of inorganic/organic or organic/organic composites with polyelectrolyte(s) as active part of the sorbent proved to be of high interest for immobilization of different pollutant types from aqueous environment, due to the large variety and high numbers of functional groups (carboxylic, sulphonic, amine, imine, hydroxyl) [125]. Immobilization efficiency and selectivity through complexation, ion exchange, electrostatic and hydrophobic interactions were linked to the nature of both functional groups and pollutants.
The nature of composite components, the cross-linking density of the synthesized material and the polyelectrolyte architecture significantly influence the sorption properties and kinetics. Thus, after the physical state of composites (shape, size, density, cross-linking degree etc.), the chemical interactions will be the second important parameter that dictates the material capacity for pollutant detection, immobilization, concentration in solid phase and subsequent releasing for a new starting sorption cycle. The sorption process of inorganic (Me2+) or organic pollutants (dyes, pharmaceuticals etc.) are driven mainly by electrostatic interactions, coordinative bonds formation, dipole-dipole or Yoshida H-bonding interactions, and ion exchange interactions. The schematic diagram of all these interactions is presented in Figure 34.
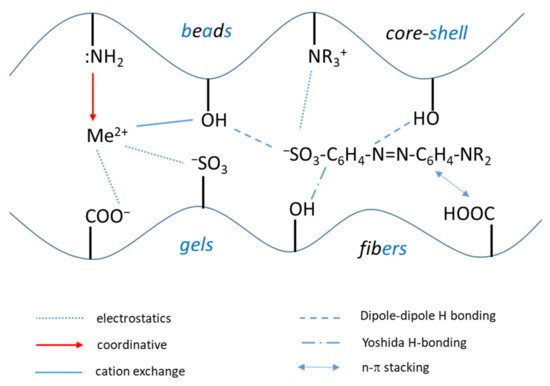

Figure 34. Principal types of interactions between inorganic (Me2+) and organic pollutants with composite polyelectrolyte sorbents functional groups.
This review brings a clear outlook on the benefits of using different moieties types as active sites of composites based polyelectrolytes for the removal by sorption mechanism of a wide range of toxic, undesired pollutants dissolved in water and wastewaters. The following subsections will be in accordance with the physical state of composite materials, such as beads, core-shells, gels, fibers and membranes, where the chemistry behind each pollutant retention is based only on sorptive mechanisms.
2.2. Beads Composites Based on Polyelectrolytes
3.2. Beads Composites Based on Polyelectrolytes
In the last years, numerous studies have been focused towards combination between organic polymers and organic/inorganic entities (see Table 1) due to their structural diversity, which can be successfully used in removal of inorganic/organic pollutants dissolved in aqueous media. Sorption processes in water treatment could require granular materials with certain size and, thus, the beads/microbeads composites based on polyelectrolytes could widen the possibilities of pollutants extraction in solid phases. Novel binary/ternary beads composites, where polyelectrolyte chain is the main component, could be mainly obtained by: (a) combination of CS with organic species (e.g., starches-g-polyacrylonitrile [53], carrageenan [31], PVAm [54], PEI [55], microcrystalline cellulose [56], carboxymethyl-β-cyclodextrin [42], QCS [20][60][20,60], citrate [52]) and/or inorganic (e.g., Fe3O4 [52], Fe [42]), (b) combination of SA with organic/inorganic entities, such as activated carbon [77], CMC [79], polyaniline [61], different types of clays (bentonite [63][78][80][63,78,80], ilite [76], kaolinite [76], montmorillonite [68][81][82][68,81,82], Fe3O4 [59][64][67][59,64,67], SiO2 [65], hydroxyapatite [66]), and (c) combination of other types of polyelectrolytes (PEI, PAA) [97] to form interpolyelectrolyte composite beads. The physico-chemical integrity of combined architectures inside the bead composite must be kept under different environmental conditions (pH, ionic strength, temperature, magnetic field, etc.); therefore, the cross-linking of organic/inorganic components is very important for beads stability/integrity and functional groups subsequent accessibility by the pollutant molecule. The cross-linking could be carried out in situ (emulsification method) during the bead formation or after the coagulation/precipitation or frozen of composite beads. The most important cross-links could be achieved by covalent or ionic cross-linking with bifunctional compounds (e.g., glutaraldehyde [53][62][53,62], ECH [20][31][54][55][96][20,31,54,55,96], poly(ethyleneglycol diglycidyl ether) [53], carbodiimide [42][52][42,52], 3-chloro-2-hydroxypropyl trimethyl ammonium chloride [60] etc.) and small ions (ionic gelation method), respectively, including Ca2+ [56][61][63][64][65][66][68][76][77][78][80][82][56,61,63,64,65,66,68,76,77,78,80,82], Ce3+ [67], Zr4+ [59], Fe3+), tripolyphoshate [54]. Bifunctional reagents (glutaraldehyde, ECH etc.) can react with primary amino groups (CS, PEI, PVAm) to form covalent cross-links, while small ions such as Ca2+, Fe3+, or Zr4+ can ionically interact with two, three, or four carboxyl groups of SA.
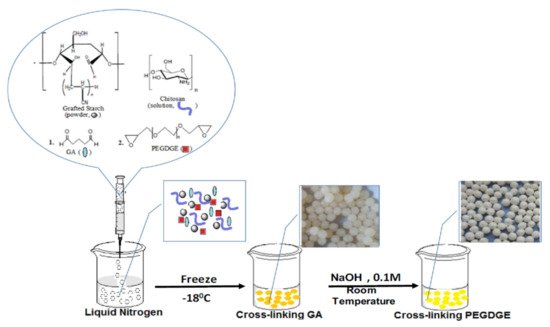
Dragan and Apopei Loghin [53] obtained biosorbents cryobeads from chitosan and starch, using glutaraldehyde and poly(ethylene glycol diglycidyl ether) as dual cross-linkers (Figure 45). It was shown that these composite cryobeads kept their integrity and sorption capacity toward three heavy metal ions (Cu2+, Ni2+ and Co2+) during five sorption/desorption cycles.

Numerous authors obtained composite beads with magnetic properties by embedding Fe3O4 or Fe inside the polymeric matrix, which can be CS/carrageenan [31], CS/carboxymethyl-β-cyclodextrin [42], carboxymethyl chitosan/chitosan/citrate [52], calcium alginate [64], cerium alginate [67] etc., feasible to be used both in batch and column sorption studies.
Magnetic composites beads based on natural polyelectrolytes have attracted the scientists’ attention due to the high sorption/selectivity capacity. Liang and co-workers [31] obtained composite beads with magnetic responsiveness, showing high sorption efficiency toward dyes (MB and CR) and heavy metal ions (Cu2+ and Cr3+). Gopalakannan and Viswanathan [67] obtained magnetic alginate composites beads by incorporating Fe3O4 into SA network followed by Ce3+ ionic gelation (Figure 56). The synthesized beads presented higher sorption capacity for chromate ions (14.29 mg/g) compared with beads without Fe3O4 (9.45 mg/g) and single Fe3O4 particles (9.72 mg/g). In this study it was shown the spontaneous and endothermic nature of chromium sorption.
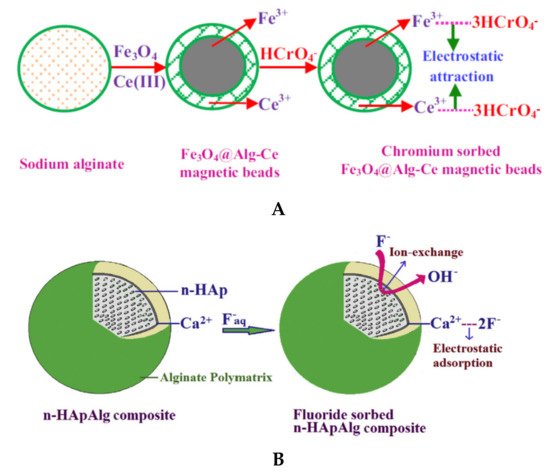

To improve the mechanical stability and porosity/accessibility inside the composite beads, different types of clays (bentonite, kaolinite, montmorillonite), SiO2, hydroxyapatite etc., have been included during the beads synthesis [63][65][66][68][76][80][63,65,66,68,76,80], where the inorganic component could act as a cross-linker agent together with small ions, such as Ca2+. Pandi and Viswanathan [66] showed that defluorination capacity of hydroxyapatite (1296 mg F−/kg) and SA beads (680 mg F−/kg) increased to 3870 mg F−/kg for SA/hydroxyapatite composite beads. Belhouchat and co-workers [63] used bentonite to obtain SA/bentonite composite beads with high sorption capacity for anionic dyes, such as MB and MO. The authors observed that both anionic and cationic dyes could be immobilized by SA composite beads with bentonite inside. The MO sorption increased with bentonite content of SA composite, while MB sorption decreased.
Uyar and co-workers [82] demonstrated that the drying method of composite beads could have a significant influence onto subsequent sorption of different types of pollutants. Composites that were deep-freezed at −21 °C presented a drastically modified morphology of beads and improved surface area and sorption capacity, compared with beads dried at room temperature. The pollutants sorption capacity and selectivity of synthesized composite beads could be drastically improved by subsequent grafting of different small molecules [98] or polymers (PEI [55], polyaniline [61]) onto the solid beads surface. The PEI beads modified with 3-chloropropanesulfonyl chloride exhibited high removal percentage for Hg2+ (>87%) and high selectivity in the presence of competing ions (Mn2+, Ni2+, Fe2+, Pb2+, Zn2+, and Cr3+) [98].
2.3. Core-Shell Composites
3.3. Core-Shell Composites
Many scientists conducted pollutant sorption studies on different types of small inorganic (SiO2, TiO2, Fe3O4, clays, minerals, etc.) and organic (active carbon, GO, biochar) solid surfaces [126]. The pollutant immobilization on a certain surface strongly depends on the nature, concentration, distribution and accessibility of material functional groups. Inorganic sorbents present very good kinetics but low pollutant sorption capacity relative to sorbent amount. Polyelectrolytes, with high number of functional groups on unit mass, present very high sorption capacities but low kinetics due to slow diffusion in the collapsed state.
The core-shell architecture could be achieved by direct deposition of different types of natural/synthetic polyelectrolytes (CS, PEI, humic acid, PAH, PVAm, PAA, PSS etc.) on 2D-cores, such as GO [21][22][23][25][115][116][21,22,23,25,115,116] and Ti3C2-MXenes [117], or 3D-cores, including Fe3O4 [57][108][122][123][126][127][57,108,122,123,126,127], SiO2 [24][28][83][90][100][101][103][109][24,28,83,90,100,101,103,109], SiO2/Fe3O4 [88][128][88,128], PS [91], FeS [129], clays [99][111][112][99,111,112], CaCO3 [102], mesoporous diatomite [89], natural fibers [107] and sand [123]. The direct deposition of the organic or organic/inorganic “shell” onto inorganic or organic “core” could be carried out in (i) one-step, by physisorption [21][89][108][111][112][21,89,108,111,112], grafting [23][83][88][100][115][117][23,83,88,100,115,117], ionic or solvent gelation/precipitation [25][27][57][69][128][25,27,57,69,128] or (ii) a multi-step procedure, such as layer-by-layer [24][90][101][102][103][109][24,90,101,102,103,109]. Using a strong polyelectrolyte (PSS), Chong and co-workers [108] obtained a stable magnetite nanoparticles with excellent dye removal efficiency (~94%). By simple PSS sorption onto Fe3O4, the dynamic light scattering and electrophoretic measurements showed a constant hydrodynamic diameter of 150 nm of the magnetic composites over 5 h measuring. This stable dispersion had 50% higher dye sorption capacity compared with bare magnetite nanoparticles. Also, Yao and co-workers [129] obtained stable core-shell colloids (~65–90 nm) based on FeS and PAA with increased sorption properties for Cr6+ sorption compared with unmodified FeS. Using direct deposition, by pH inversion precipitation of carboxymethylchitosan onto unmodified and modified silica particles, Aden and collaborators [83] obtained core-shell composites with excellent sorption properties for Ni2+ (Figure 67). After drying at 100 °C for 24 h, the composite particles were used without further modification steps as Ni2+ ion sorbent at pH 5 and 7.
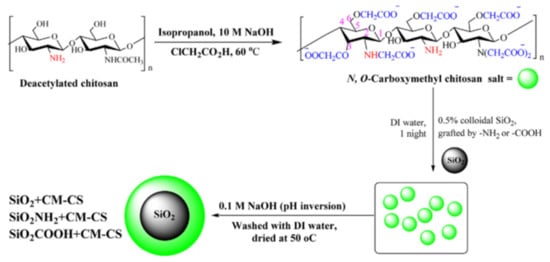

Figure 67. Synthesis of a core-shell composite based on carboxymethylchitosan (CM-CS) and silica particles (reprinted with permission from ref. [83]).
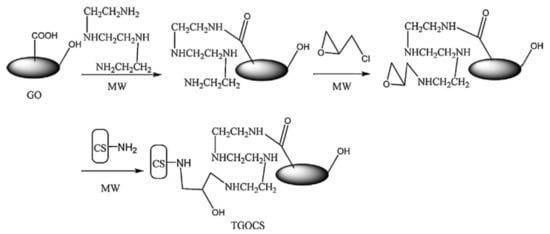
Sometimes, due to harsh environment conditions (extreme pH, high ionic strength, temperature, etc.), which can delaminate the “shell” of composite, the direct grafting of polymeric chains to the solid “core” or “shell” cross-linking after or during deposition, must be carried out. For more stable composites over wide ranges of environment stimuli, many authors anchor polyelectrolyte chains covalently to the inorganic core, which contains a linker molecule on surface [88][100][115][88,100,115]. In this way, the new created core-shell composite is more stable in aqueous media and more effective in sorption of different pollutants. The immobilization of “shell” around the “core” could be achieved by chemical cross-linking with bifunctional compounds, such as glutaraldehyde [89][102][103][109][115][89,102,103,109,115], epichlorohydrin [23], α,α’-dichloro-p-xylene [24], phtaldialdehyde [91]. Ge and Ma [23] obtained CS/GO composite microparticles by microwave irradiation method using GO, triethylenetetraamine, ECH and CS (Figure 78). Sorption of Cr(VI) onto composite particles showed high values, reaching 219 mg/g at pH 2. The results obtained by batch experiments showed that sorption capacity of synthesized composite increased with temperature and the sorbent material could be recyclable.
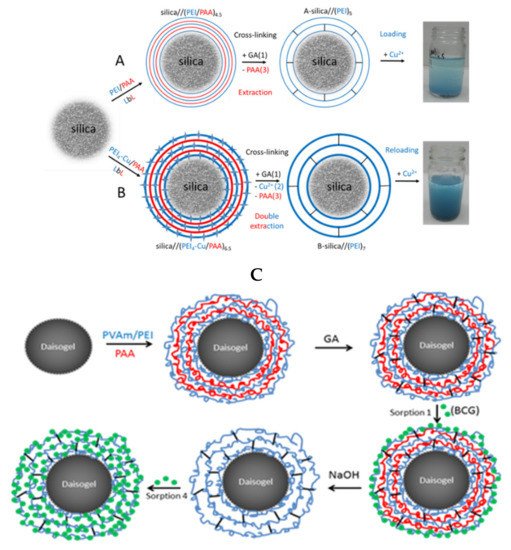
Bucatariu and co-workers used selective cross-linking to obtain core-shell silica composites based on PEI, PVAm, polylysine, and PAH. This type of composites has been utilized in the removal of dyes [109] and heavy metal ions [90][101][103][90,101,103] from real and simulated waters. The layer-by-layer technique involved in these studies allowed a controlled deposited polyelectrolyte amount onto spherical silica particles. Furthermore, the subsequent glutaraldehyde cross-linking stabilized the polycation layer onto each individual solid particle. To increase the multilayer flexibility and functional groups accessibility toward pollutant molecules, the polyanion has been removed from the cross-linked multilayer in extreme basic medium, as it can be seen in Figure 89. The fast kinetic of Cu2+ sorption and high sorbed amount of anionic dyes (BCG, CR) after polyanion extraction, confirmed the polyelectrolyte multilayer stability and flexibility after cross-linking and polyanion extraction. Based on distribution parameter and relative atomic concentration of elements on surface, the authors demonstrated that silica/(PEI)10 composite particles can clean (>95%) a simulated water contaminated with four heavy metal ions (Cu2+, Co2+, Ni2+, Cd2+), if the ratio between number of composite functional groups and number of ions is higher than ~9 (non-competitive regime). The competitive sorption between different metal ions showed that the composite had high selectivity for Cu2+. Subsequent chemical modification of stable cross-linked core-shell composites with small molecules, i.e., disodium ethylenediamine tetraacetate (EDTA-2Na), increased the sorption capacities by creation of new functional groups onto core-shell surface.