Heavy metal contamination affects lives with concomitant environmental pollution, and seaweed has emerged as a remedy with the ability to save the ecosystem, due to its eco-friendliness, affordability, availability, and effective metal ion removal rate. Heavy metals are intrinsic toxicants that are known to induce damage to multiple organs, especially when subjected to excess exposure. With respect to these growing concerns, this review presents the preferred sorption material among the many natural sorption materials. The use of seaweeds to treat contaminated solutions has demonstrated outstanding results when compared to other materials. The sorption of metal ions using dead seaweed biomass offers a comparative advantage over other natural sorption materials. This article summarizes the impact of heavy metals on the environment, and why dead seaweed biomass is regarded as the leading remediation material among the available materials.
- heavy metals
- seaweed
- biosorption
- aqueous solution
- remediation
1. Structure and Classification of Seaweed
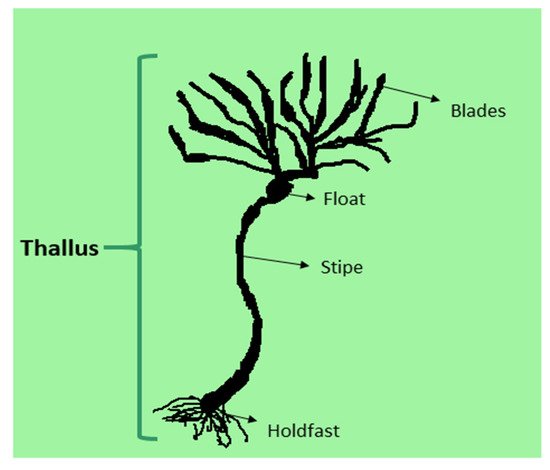
| Common Name (Phylum) | Body Form | Size | Pigments | Colour Composition | Cell Walls |
|---|---|---|---|---|---|
| Brown algae (Phaeophyta) | Multicellular | 60 cm–60 m | Chlorophyll, Fucoxanthin, and several other xanthophylls | Golden-brown, Greenish-brown | Cellulose, Alginate, Fucoidan |
| Red algae (Rhodophyta) | Multicellular | 50 cm–2 m | Chlorophyll, Phycocyanin, Phycoerythrin, and several xanthophylls | Brownish red, Purple | Cellulose, Xylans, Galactans |
| Green algae (Chlorophyta) | Unicellular, Colonial, Filamentous, Multicellular | 1–1000 μm | a and b Chlorophyll and several xanthophylls | Green | Cellulose Hydroxyl –proline glucosides β- xylans, β-mannans |
2. Seaweed: Metal Ion Biosorption Material
3. Various Natural Materials Used for Sorption
| Materials Used | Heavy Metals | References |
|---|---|---|
| Polymers | Fe and Cr | [74][21] |
| Sawdust and tree barks | Hg, Pb, and Zn | [75][22] |
| Electronic waste along with galvanic wastes | Cu, Ni, Mn, Pb, Sn | [76][23] |
| charcoal: | Cr(III) | [77][24] |
| Clay | Cr(III) | [78][25] |
| Fungi | Cr, Fe | [79][26] |
| Dead biomass | Cr | [80][27] |
| Peat moss | Cr, Fe | [81][28] |
| Peanut shells, Rice husk, Straw, and walnut cover | Cr, Cu, Ni | [82][29] |
| Cocoa shell | Al, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, and Zn | [83][30] |
| Coconut husk | Cr, As | [82][29] |
| Caol and fly ashes | Cr, Cu, Ni | [84][31] |
| Banana pith and peels | Ni, Pb | [85][32] |
| Cassava fiber | Pb, Co | [86][33] |
| Chicken feathers | Al, As | [87][34] |
| Sheep manure wastes | Ca, Cd | [88][35] |
| Sunflower | Co, Cr | [89][36] |
| Rice byproducts | Cu, Fe | [90][37] |
| Orange peels | Cu, Fe, Hg | [91][38] |
| Palm kernel fiber | Fe, Hg | [82][29] |
| Grape stalks | Cr, Fe, Hg | [92][39] |
References
- Mahadevan, K. Seaweeds: A sustainable food source. In Seaweed Sustainability; Elsevier BV: Manchester, UK, 2015; pp. 347–364.
- Collins, K.G. An investigation of the prebiotic potential and gut health benefits of Irish seaweeds. Univ. Coll. Cork 2017, 371, 31–35.
- Gade, R.; Tulasi, M.S.; Bhai, V.A. Seaweeds: A novel biomaterial. Int. J. Pharm. Pharm. Sci. 2013, 5, 975–1491.
- Harbo, J.R.; Harris, J.W. Heritability in Honey Bees (Hymenoptera: Apidae) of Characteristics Associated with Resistance to Varroa jacobsoni(Mesostigmata: Varroidae). J. Econ. Entomol. 1999, 92, 261–265.
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330.
- Bittner, L.; Payri, C.; Couloux, A.; Cruaud, C.; De Reviers, B.; Rousseau, F. Molecular phylogeny of the Dictyotales and their position within the Phaeophyceae, based on nuclear, plastid and mitochondrial DNA sequence data. Mol. Phylogenetics Evol. 2008, 49, 211–226.
- Romera, D.E.; González, F.; Ballester, A.; Blázquez, M.L.; Muñoz, J.A. Biosorption with Algae: A Statistical Review. Crit. Rev. Biotechnol. 2006, 26, 223–235.
- Yalçın, S.; Sezer, S.; Apak, R. Characterization and lead (II), cadmium (II), nickel (II) biosorption of dried marine brown macro algae Cystoseira barbata. Environ. Sci. Pollut. Res. 2012, 19, 3118–3125.
- Sheng, P.X.; Ting, Y.-P.; Chen, J.P.; Hong, L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms. J. Colloid Interface Sci. 2004, 275, 131–141.
- Adamu, C.; Nganje, T.; Edet, A. Heavy metal contamination and health risk assessment associated with abandoned barite mines in Cross River State, southeastern Nigeria. Environ. Nanotechnol. Monit. Manag. 2015, 3, 10–21.
- Badruddoza, A.Z.M.; Shawon, Z.B.Z.; Tay, W.J.D.; Hidajat, K.; Uddin, M.S. Fe3O4/cyclodextrin polymer nanocom-posites for selective heavy metals removal from industrial wastewater. Carbohydr. Polymers 2013, 91, 322–332.
- Turan, N.G.; Mesci, B. Use of Pistachio Shells as an Adsorbent for the Removal of Zinc(II) Ion. CLEAN—Soil Air Water 2011, 39, 475–481.
- Pozdniakova, T.A.; Mazur, L.P.; Boaventura, R.A.; Vilar, V.J. Brown macro-algae as natural cation exchangers for the treatment of zinc containing wastewaters generated in the galvanizing process. J. Clean. Prod. 2016, 119, 38–49.
- Mata, Y.; Blázquez, M.; Ballester, A.; González, F.; Muñoz, J.A. Characterization of the biosorption of cadmium, lead and copper with the brown alga Fucus vesiculosus. J. Hazard. Mater. 2008, 158, 316–323.
- Michalak, I.; Chojnacka, K. Interactions of metal cations with anionic groups on the cell wall of the macroalga Vaucheria sp. Eng. Life Sci. 2010, 10, 209–217.
- Gupta, V.; Rastogi, A. Biosorption of lead from aqueous solutions by green algae Spirogyra species: Kinetics and equilibrium studies. J. Hazard. Mater. 2008, 152, 407–414.
- Mehta, S.K.; Gaur, J.P. Use of Algae for Removing Heavy Metal Ions From Wastewater: Progress and Prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152.
- Errasquín, E.L.; Vázquez, C. Tolerance and uptake of heavy metals by Trichoderma atroviride isolated from sludge. Chemosphere 2003, 50, 137–143.
- Bishnoi, N.R.; Kumar, R.; Kumar, S.; Rani, S. Biosorption of Cr(III) from aqueous solution using algal biomass spirogyra spp. J. Hazard. Mater. 2007, 145, 142–147.
- Ebrahimi, B.; Shojaosadati, S.; Ranaie, S.; Mousavi, S. Optimization and evaluation of acetylcholine esterase immobilization on ceramic packing using response surface methodology. Process. Biochem. 2010, 45, 81–87.
- Pathe, P.P.; Nandy, T.; Kaul, S.N.; Szpyrokwicz, L. Chromium recovery from chrome tan wastewater. Int. J. Environ. Stud. 1996, 51, 125–145.
- Lichtfouse, E.; Elbisser, B. 2nd European Meeting on Environmental Chemistry. In Proceedings of the 2nd European Meeting on Environmental Chemistry, Dijon, France, 12–15 December 2001; p. 273.
- Vegliò, F.; Quaresima, R.; Fornari, P.; Ubaldini, S. Recovery of valuable metals from electronic and galvanic industrial wastes by leaching and electrowinning. Waste Manag. 2003, 23, 245–252.
- Dahbi, S.; Azzi, M.; Saib, N.; De la Guardia, M.; Faure, R.; Durand, R. Removal of trivalent chromium from tannery waste waters using bone charcoal. Anal. Bioanal. Chem. 2002, 374, 540–546.
- Park, S.-J.; Jung, W.-Y. Removal of chromium by activated carbon fibers plated with copper metal. Carbon Lett. 2001, 2, 15–21.
- Bai, R.; Abraham, T. Studies on enhancement of Cr(VI) biosorption by chemically modified biomass of Rhizopus nigricans. Water Res. 2002, 36, 1224–1236.
- Chong, K.H.; Volesky, B. Metal biosorption equilibria in a ternary system. Biotechnol. Bioeng. 1996, 49, 629–638.
- Lee, S.-J.; Park, J.H.; Ahn, Y.; Chung, J.W. Comparison of Heavy Metal Adsorption by Peat Moss and Peat Moss-Derived Biochar Produced Under Different Carbonization Conditions. Water Air Soil Pollut. 2015, 226, 9.
- Ofomaja, A.; Ho, Y.-S. Effect of pH on cadmium biosorption by coconut copra meal. J. Hazard. Mater. 2007, 139, 356–362.
- Meunier, N.; Laroulandie, J.; Blais, J.; Tyagi, R. Cocoa shells for heavy metal removal from acidic solutions. Bioresour. Technol. 2003, 90, 255–263.
- Tan, L.C.; Choa, V.; Tay, J.H. The Influence of pH on Mobility of Heavy Metals from Municipal Solid Waste Incinerator Fly Ash. Environ. Monit. Assess. 1997, 44, 275–284.
- Foday Jr, E.H.; Ramli, N.A.S.; Ismail, H.N.; Malik, N.A.; Basri, H.F.; Aziz, F.S.A.; Nor, N.S.M.; Jumhat, F. Municipal solid waste characteristics in Taman Universiti, Skudai, Johore, Malaysia. J. Adv. Res. Des. 2017, 38, 13–20.
- Abia, A.; Asuquo, E. Lead (II) and nickel (II) adsorption kinetics from aqueous metal solutions using chemically modified and unmodified agricultural adsorbents. Afr. J. Biotechnol. 2006, 5, 1475–1482.
- Al-Asheh, S.; Banat, F.; Al-Rousan, D. Adsorption of Copper, Zinc and Nickel Ions from Single and Binary Metal Ion Mixtures on to Chicken Feathers. Adsorpt. Sci. Technol. 2002, 20, 849–864.
- Abu Al-Rub, F.A.; Kandah, M.; Al-Dabaybeh, N. Competitive Adsorption of Nickel and Cadmium on Sheep Manure Wastes: Experimental and Prediction Studies. Sep. Sci. Technol. 2003, 38, 483–497.
- Özdemir, N.; Horn, R.; Friedt, W. Construction and characterization of a BAC library for sunflower (Helianthus annuus L.). Euphytica 2004, 138, 177–183.
- Ajmal, M.; Rao, R.A.K.; Anwar, S.; Ahmad, J.; Ahmad, R. Adsorption studies on rice husk: Removal and recovery of Cd(II) from wastewater. Bioresour. Technol. 2003, 86, 147–149.
- Ajmal, M.; Rao, R.A.K.; Ahmad, R.; Ahmad, J. Adsorption studies on Citrus reticulata (fruit peel of orange): Removal and recovery of Ni(II) from electroplating wastewater. J. Hazard. Mater. 2000, 79, 117–131.
- Fiol, N.; Villaescusa, I.; Martínez, M.; Miralles, N.; Poch, J.; Serarols, J. Sorption of Pb (II), Ni (II), Cu (II) and Cd (II) from aqueous solution by olive stone waste. Sep. Purif. Technol. 2006, 50, 132–140.
- Kapoor, A.; Viraraghavan, T. Heavy metal biosorption sites in Aspergillus niger. Bioresour. Technol. 1997, 61, 221–227.
- Vaishya, R.; Prasad, S. Adsorption of copper (II) on sawdust. Indian J. Environ. Prot. 1991, 11, 284–289.
- Vázquez, G.; Antorrena, G.; González-Álvarez, J.; Doval, M. Adsorption of heavy metal ions by chemically modified Pinus pinaster bark. Bioresour. Technol. 1994, 48, 251–255.
- Srivastava, S.K.; Gupta, V.K.; Mohan, D. Removal of Lead and Chromium by Activated Slag—A Blast-Furnace Waste. J. Environ. Eng. 1997, 123, 461–468.
- Bailey, S.E.; Olin, T.J.; Bricka, R.; Adrian, D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479.
- Keskinkan, O.; Goksu, M.; Basibuyuk, M.; Forster, C. Heavy metal adsorption properties of a submerged aquatic plant (Ceratophyllum demersum). Bioresour. Technol. 2004, 92, 197–200.
- Gardea-Torresdey, J.; Peralta-Videa, J.; Montes, M.; de la Rosa, G.; Corral-Diaz, B. Bioaccumulation of cadmium, chromium and copper by Convolvulus arvensis L.: Impact on plant growth and uptake of nutritional elements. Bioresour. Technol. 2004, 92, 229–235.
- Dumitriu, S. Polysaccharides as Biomaterials. In Polymeric Biomaterials, Revised and Expanded; CRC Press: New York, NY, USA, 2001; pp. 15–76.
- Brown, P.; Gill, S.; Allen, S.J. Determination of optimal peat type to potentially capture copper and cadmium from solu-tion. Water Environ. Res. 2001, 73, 351–362.
- Celis, R.; Hermosín, M.C.; Cornejo, J. Heavy Metal Adsorption by Functionalized Clays. Environ. Sci. Technol. 2000, 34, 4593–4599.
