The circadian clock is a biological clock that regulates processes in cells and whole organs, contributing to dynamic physiology over the 24 hour period.
Intermittent hypoxia (IH) is defined as alternating periods of hypoxia and normoxia. It is associated with multiple respiratory conditions such as chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA).
- intermittent hypoxia
- circadian rhythms
1. Introduction
2. IH Alters Tissue-Specific Circadian Rhythms of Canonical Clock Genes
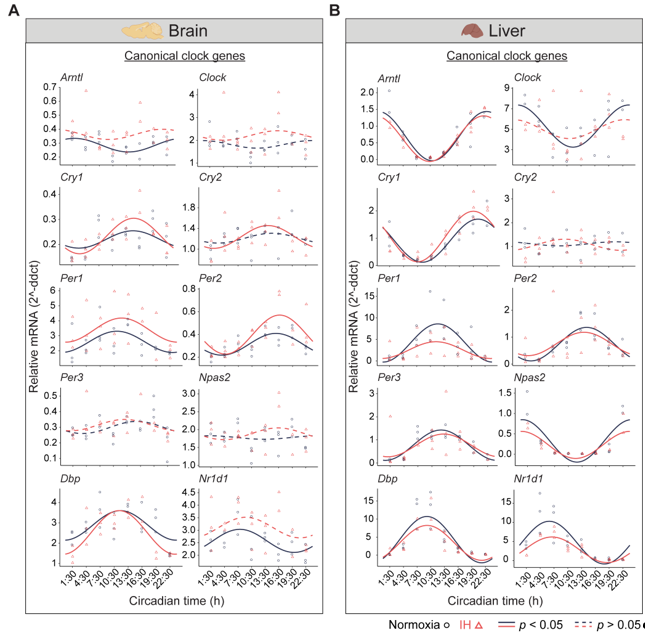

Figure 1. Circadian rhythms of canonical clock genes. Left (brain) and right (liver) panels represent the expression of canonical clock genes over 24h on the second day of constant dark after exposure to a normoxia (circles; black) and IH (triangles; red). Solid and dashed lines represent rhythmic (
p < 0.05) and non-rhythmic (
p > 0.05) expression patterns, respectively.
3. The Clock in the Liver Is More Stable against IH Compared to the Clock in the Brain
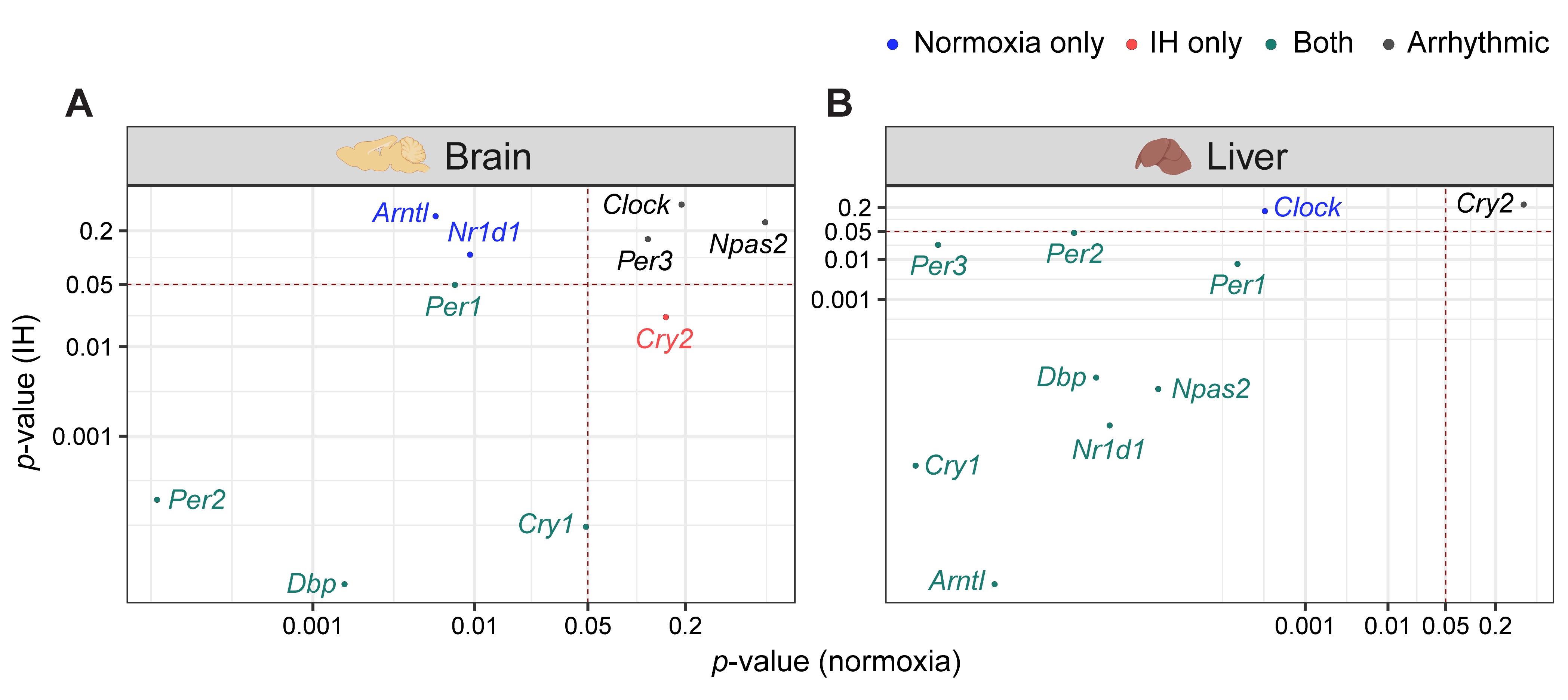
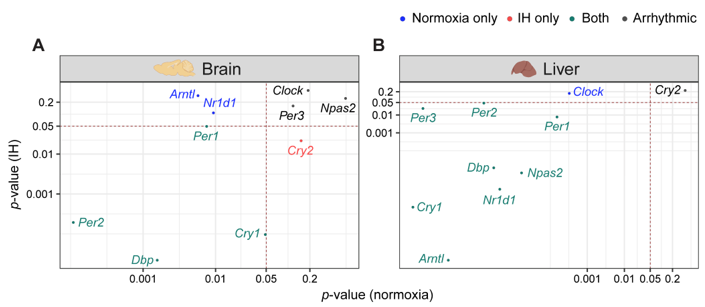
4. Conclusion
References
- Wagner, P.D. The biology of oxygen. Eur. Respir. J. 2008, 31, 887–890.
- Tripathi, A.; Melnik, A.V.; Xue, J.; Poulsen, O.; Meehan, M.J.; Humphrey, G.; Jiang, L.; Ackermann, G.; McDonald, D.; Zhou, D.; et al. Intermittent hypoxia and hypercapnia, a hallmark of obstructive sleep apnea, alters the gut microbiome and metabolome. mSystems 2018, 3, e00020-18.
- Zieliński, J. Effects of intermittent hypoxia on pulmonary haemodynamics: Animal models versus studies in humans. Eur. Respir. J. 2005, 25, 173–180.
- Neubauer, J.A. Invited review: Physiological and pathophysiological responses to intermittent hypoxia. J. Appl. Physiol. 2001, 90, 1593–1599.
- Adamovich, Y.; Ladeuix, B.; Sobel, J.; Manella, G.; Neufeld-Cohen, A.; Assadi, M.H.; Golik, M.; Kuperman, Y.; Tarasiuk, A.; Koeners, M.P.; et al. Oxygen and carbon dioxide rhythms are circadian clock controlled and differentially directed by behavioral signals. Cell Metab. 2019, 29, 1092–1103.e3.
- Adamovich, Y.; Ladeuix, B.; Golik, M.; Koeners, M.P.; Asher, G. Rhythmic oxygen levels reset circadian clocks through HIF1α. Cell Metab. 2017, 25, 93–101.
- Bersten, D.C.; Sullivan, A.E.; Peet, D.J.; Whitelaw, M.L. bHLH-PAS proteins in cancer. Nat. Rev. Cancer 2013, 13, 827–841.
- O’Connell, E.J.; Martinez, C.-A.; Liang, Y.G.; Cistulli, P.A.; Cook, K.M. Out of breath, out of time: Interactions between HIF and circadian rhythms. Am. J. Physiol. Cell Physiol. 2020, 319, C533–C540.
- Manella, G.; Aviram, R.; Bolshette, N.; Muvkadi, S.; Golik, M.; Smith, D.F.; Asher, G. Hypoxia induces a time- and tissue-specific response that elicits intertissue circadian clock misalignment. Proc. Natl. Acad. Sci. USA 2020, 117, 779–786.
- Wu, G.; Lee, Y.Y.; Gulla, E.M.; Potter, A.; Kitzmiller, J.; Ruben, M.D.; Salomonis, N.; Whitsett, J.A.; Francey, L.J.; Hogenesch, J.B.; et al. Short-term exposure to intermittent hypoxia leads to changes in gene expression seen in chronic pulmonary disease. Elife 2021, 10, e63003.
- Casas, A.I.; Geuss, E.; Kleikers, P.W.M.; Mencl, S.; Herrmann, A.M.; Buendia, I.; Egea, J.; Meuth, S.G.; Lopez, M.G.; Kleinschnitz, C.; et al. NOX4-dependent neuronal autotoxicity and BBB breakdown explain the superior sensitivity of the brain to ischemic damage. Proc. Natl. Acad. Sci. USA 2017, 114, 12315–12320.
- Leach, R.M.; Treacher, D.F. Oxygen transport-2. Tissue hypoxia. BMJ 1998, 317, 1370–1373.
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224.
- Manella, G.; Sabath, E.; Aviram, R.; Dandavate, V.; Ezagouri, S.; Golik, M.; Adamovich, Y.; Asher, G. The liver-clock coordinates rhythmicity of peripheral tissues in response to feeding. Nat. Metab. 2021, 3, 829–842.
- Pizarro, A.; Hayer, K.; Lahens, N.F.; Hogenesch, J.B. CircaDB: A da-tabase of mammalian circadian gene expression profiles. Nucleic Acids Res. 2013, 41, D1009–D1013.
- Albrecht, U. Timing to perfection: The biology of central and peripheral circadian clocks. Neuron 2012, 74, 246–260.
