Waste cooking oil (WCO) is considered a potential bio-based waste material because it can lead to multiple topologies of the product. WCO is generated after frying activities, and the rising population has increased its quantity due to the increased demand for food. WCO is related to the vegetable oil family and mainly arises from the kitchen and food industry.
- waste cooking oil
- transesterification
- sustainability
- free fatty acid
- asphalt binder
- rheological properties
1. Introduction
Amongst many global issues, the rapid growth of cities and the increasing number of vehicles have dramatically enhanced the demand for pavement construction and maintenance [1]. Around 95% of the world’s roads are made of flexible pavement, and asphalt binder serves as a conventional material for pavement construction [2]. Asphalt binder is a byproduct of crude oil, which is well known as a non-renewable resource. A dramatic increase in the price of petroleum-based binders, due to the depletion of petroleum reserves, forces road authorities to explore alternative materials. Currently, the global practice is moving towards a sustainable, economical, and green environment. The scientific community is interested in optimizing bio resources for renewability, sustainability, and local production in the pavement industry, due to reduced costs and lower energy consumption.
Biomass is the primary source of bio material, whereas biomass refers to living things that store solar energy, such as plants, animals, and microorganisms [3]. Among various forms of biomaterial, bio-oil is a widely accessible material and is categorized as waste oil from the forest/agriculture industry, animal, and residual. Hence, modifying the asphalt binder with bio-oil significantly minimizes the issues regarding non-renewability and fluctuation of binder cost [4]. The compatibility and likelihood of bio-oil modified asphalt are somewhat satisfactory, and its quality differs with source and the content [5]. However, the addition of agricultural waste oil (5–10 wt.%) improves the bio asphalt viscosity and low-temperature properties, whereas there is marginal improvement in the high-temperature properties [6][7][6,7]. Similarly, the waste wood oil-based asphalt mixture improved the anti-fatigue and crack resistance properties [8]. Conversely, animal waste oil is commonly utilized less than 10 wt.% by asphalt to achieve better performance [9][10][9,10]. The addition of animal waste based bio-oil reduces the viscosity and stability of asphalt at higher temperatures and increases low-temperature crack resistance [1]. Yet the agriculture and animal-based oils are subjected to time-consuming and expensive refining procedures, such as pyrolysis and hydrothermal liquefaction [3][11][3,11]. In this way, the current implementation of bio-oil to modify asphalt binder is mainly focused on plant-based oil such as waste cooking oil/waste vegetable oil, as these soft oils are a ready substrate with lower production cost [3][12][13][14][3,12,13,14].
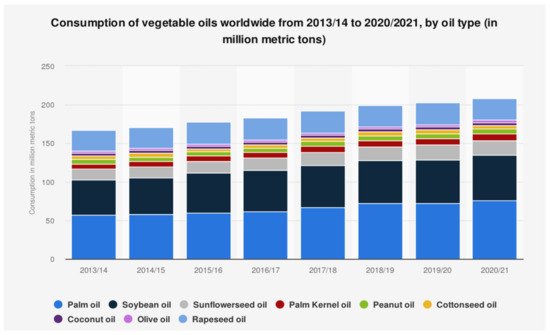
Waste cooking oil (WCO) is considered a potential bio-based waste material because it can lead to multiple topologies of the product [15]. WCO is generated after frying activities, and the rising population has increased its quantity due to the increased demand for food. WCO is related to the vegetable oil family and mainly arises from the kitchen and food industry. The global consumption of vegetable oil in 2021 has reached 209.14 million metric tons, and each year consumption has increased by 2% ( Figure 1 ). In contrast, a significant problem regarding the proper disposal and collection of WCO has still gone unsolved for a long time. The lack of awareness, facilities, and improper disposal of WCO are leading to environmental, ecological, and municipal problems [16][17][16,17]. Hence, by heavily consuming oil and fats, many countries face sewer blockage problems associated with an increased annual cost to remove blockage [18].
Azahar et al. [20][25] investigated the effect of acid value and water content on asphalt binder modification and revealed that chemically treated WCO with lower acid values and water content showed better high-temperature performance. Although, the physical performance of treated WCO was still below the control binder and compromised. To overcome the deficiencies encountered by the WCO-modified binder, researchers recently utilized additional modifiers such as waste polymers, palm oil fuel ash (POFA), and nano clay (NC). It was concluded that WCO-modified binder with other modifiers could withstand hot climate regions. Moreover, a higher percentage of WCO can be used along with additional modifiers to enhance the performance while ensuring a more sustainable environment [21][22][23][29,30,31].
2. Production of Waste Cooking Oil
The vegetable oils market is dominated by palm and soybean oils, which account for 36% and 28% of the market. Other vegetable oil fractions include sunflower (9.09%), rape (13.2%), peanut (2.95%), corn (2.1%), coconut (1.75%), olive (1.48%), and sesame (1.1%) [17]. Amongst all continents, Asia is on top of the list for highest production of vegetable oil. Domestically, edible oil is used for frying purposes, whereas food processing industries and fast food companies largely use oil [24][32]. Frying is a process in which food is fried at a temperature between 150–200 °C in the presence of moisture, anti-oxidants, and pro-oxidants [25][33]. During frying, various reactions occur, such as hydrolysis, polymerization, isomerization, and oil decomposition. After a frying operation, the leftover oil is considered waste material and named waste cooking oil or used cooking oil [26][34]. The rapidly growing world’s population has dramatically increased the food demand that ultimately affects the production of WCO. Recent studies estimate that the generation of waste cooking oil is almost 20–32% of total edible oil consumption [17][27][17,35]. China and India are the most populated countries and produce the most WCO, around 5.6 million tons and 1.135 million tons per year. Canada, Demark, Spain, Italy, Japan, South Korea, Malaysia, and the United Kingdom have average generations, ranging from 0.1–0.5 million tons per year [28][36]. The other remaining countries produce no more than 0.1 million tons per year, and as per capita production of WCO, South Korea is on top of the list, followed by EU countries [17].
3. Free Fatty Acid in Waste Cooking Oil
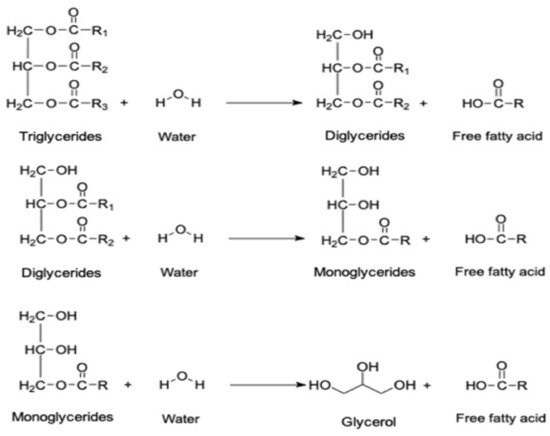
Edible oil undergoes chemical and physical changes during frying activities, and the quality of the oil degrades. The steam generation during frying reacts with the oxygen and water in the food, initiates the hydrolysis process, and produces the FFA. The concentration of the FFA varies and depends on diverse conditions, such as heating temperature, moisture content, and storage time. Thus, during the additional reaction time the triglyceride bonds break down and produce FFA, including glycerol, diglyceride, and monoglycerides, as shown in Figure 2 . Furthermore, at a temperature above 150 °C, the glycerol accelerates the hydrolysis process, leading to further FFA production [29][44]. As the degradation process continues, the production of FFA accelerates, which ultimately decreases the unsaturation level of the cooking oil. Hence, the oil becomes no longer suitable for further frying and needs to be discarded as waste material [30][45]. The WCO quality can be monitored by the acid value parameter, which identifies the concentration of FFA and the amount of water content [19][31][19,46]. However, researchers have utilized the gas chromatography–mass spectrometry (GC–MS) technique to analyze the concentration of FFA in WCO. The acidic compounds of WCO are categorized as saturated, monounsaturated, and polyunsaturated compounds. The chemical composition of WCO studied by Azahar et al. [32][47] indicated that palmitic acid, stearic acid, and meristic acid are examples of saturated fatty acids, whereas monounsaturated fatty acids contain oleic acid and cis-11-eicosenoic acid. Table 1 indicates that the main components of WCO are oleic acid, palmitic acid, and linoleic acid, which account for 43.67%, 38.35%, and 11.39%, respectively [19][33][19,24].
| Formulation of Fatty Acids | Type of Free Fatty Acid | Possible % in Waste Cooking Oil (WCO) | Type of Saturation |
|---|---|---|---|
| C18:1 (Cis 9) | Oleic Acid | 43.67 | Unsaturated |
| C16:0 | Palmitic acid | 38.35 | Saturated |
| C18:2 (Cis) | Linoleic acid | 11.39 | Unsaturated |
| C18:0 | Stearic acid | 4.33 | Saturated |
| C14:0 | Myristic acid | 1.03 | Saturated |
| C18:3 alpha | ɣ- Linolenic acid | 0.37 | Unsaturated |
| C12:0 | Lauric acid | 0.34 | Saturated |
| C18:2 t | Linolenic acid | 0.29 | Unsaturated |
| C20:1 | Cis-11-Eicosenoic acid | 0.16 | Unsaturated |
| C21:0 | Heneicosanoic acid | 0.08 | Saturated |
| TOTAL | 100 |
4. Available Technologies to Minimize FFA from Waste Cooking Oil
The simplicity of distillation deserves to be described, as it is the most used physical method. The method aims to reduce volatile compounds and water content by taking advantage of the fact that FFA has higher volatility than triglycerides. This process is carried out by simply heating filtered WCO in boiling kettles under vacuum conditions for about ≤90 min [27][35]. Table 2 summarizes key parameters for acidity and water reduction by means of the distillation process. Thus, this process purifies WCO of volatile compounds and water content up to the maximum level. Besides this, another consequence is eliminating other volatile compounds such as ketones, aldehydes, sulfur, and nitrogen. The removal of these undesirable compounds by distillation further improves the quality of WCO [27][35].
| Reference | Temperature (°C) | Reduction in Water Content (%) | Reduction in FFA (%) | |
|---|---|---|---|---|
| [34] | [53] | 220 | From 1.15% to 0.062% | From 29% to 2% |
| [35] | [52] | 200–280 | From 5% to 0.1% | Less than 0.5% |
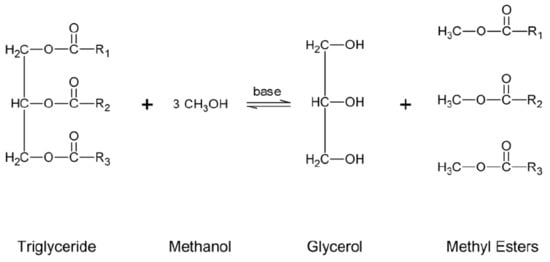
Transesterification is also known as alcoholysis, and it is the best conventional method for reducing the acid value of WCO by converting the triglycerides of an oil into ester by direct reaction with alcohols. A variety of alcohols can be used, such as methanol, ethanol, butanol, and propanol. Methanol is a more feasible option due to its wide availability, low cost, and quick reaction with triglycerides [36][59]. Generally, a 3:1 molar ratio of alcohol and oil is needed in the transesterification reaction to complete the reaction ( Figure 3 ). The kinetic analysis of the transesterification process is highly challenging, since the reaction is under an equilibrium process. Alcohol must be present in excess in the reaction mixture to shift the reaction towards the formation of the products [37][60]. However, to accelerate the transesterification reaction, commonly used catalysts are sodium hydroxide (NaOH) and potassium hydroxide (KOH). These catalysts are highly demanded for several reasons: cheap price, wide availability, and maximum yield at minimal time duration [38][61]. KOH catalyst reacts faster than NaOH due to strong bonding with alcohol. Tomasevic and Siler-Marinkovic [39][38] investigated the effect of KOH and NaOH as a catalyst; the result concluded that higher conversion of triglycerides was obtained at a 6:1 molar ratio of methanol to oil, a 90 min reaction time, 1% KOH, and 25 °C temperature. On the other hand, ester covers a wide range of end products and can be utilized in different applications, such as biodiesel production, asphalt industry, detergent, and cosmetics [20][40][25,41].
However, the ultimate maximum ester application of the base catalyst in transesterification has certain limitations, as this process is susceptible to water content and FFA presence in WCO. A higher percentage of FFA reacts with the catalyst, whereas water content partially converts the reaction to saponification, and excessive soap may foam a semi-solid mixture at room temperature, which creates problems during the separation process [37][42][60,63]. Thus, the base catalyst cannot tolerate higher FFA content in WCO, and the desirable range is from less than 0.5 to 1%, as shown in Table 3 .
| Author/Reference | Recommended FFA (%) | |
|---|---|---|
| [36] | [59] | Less than 1 |
| [43] | [64] | Less than 0.5 |
| [44] | [65] | Less than 0.5 |
| [45] | [66] | Less than 2 |
| [46] | [67] | Less than 2 |
| [20] | [25] | Less than 1 |
In recent years, many researchers have been interested in electromagnetic waves utilization. Hence, microwaves are one example of accelerating chemical reactions, such as transesterification/esterification [47][70]. Microwaves can effectively transfer heat to the sample, and this energy completes the reaction. However, in conventional processes, heat is not distributed uniformly, resulting in more energy and time-consumption. Thus, the heat transfer mechanism is more effective than traditional heating [48][71]. Further advantages of microwave irradiation for treating waste oil include a lower alcohol to oil molar ratio, being environmentally friendly, water content removal, and an immediate decrease in FFA [42][49][63,72]. Microwave transesterification of WCO with a high FFA content indicated additional benefits in the separation and purification process between treated oil and residues compared to the conventional method [50][73]. Transesterification of WCO by sodium hydroxide as the catalyst (NaOH) was carried out in a domestic microwave (800 W) under varying parameters, such as molar to ethanol ratio and reaction time. The result indicated that the conversion time of FFA to reduce the acid value of the oil by microwaves is 10 times shorter than the conventional method [48][71].
