The renin–angiotensin system (RAS), an essential enzymatic cascade involved in maintaining blood pressure and electrolyte balance, is involved in the pathogenicity of COVID-19, since the angiotensin-converting enzyme II (ACE2) acts as the cellular receptor for SARS-CoV-2 in many human tissues and organs. In fact, the viral entrance promotes a downregulation of ACE2 followed by RAS balance dysregulation and an overactivation of the angiotensin II (Ang II)–angiotensin II type I receptor (AT1R) axis, which is characterized by a strong vasoconstriction and the induction of the profibrotic, proapoptotic and proinflammatory signalizations in the lungs and other organs. This mechanism features a massive cytokine storm, hypercoagulation, an acute respiratory distress syndrome (ARDS) and subsequent multiple organ damage.
- SARS-CoV-2
- COVID-19
- ACE-2
- Ang II/AT1R axis
- RAS imbalance
- cytokine storm
- underlying diseases
- genetic polymorphisms
- host susceptibility factors
1. Introduction
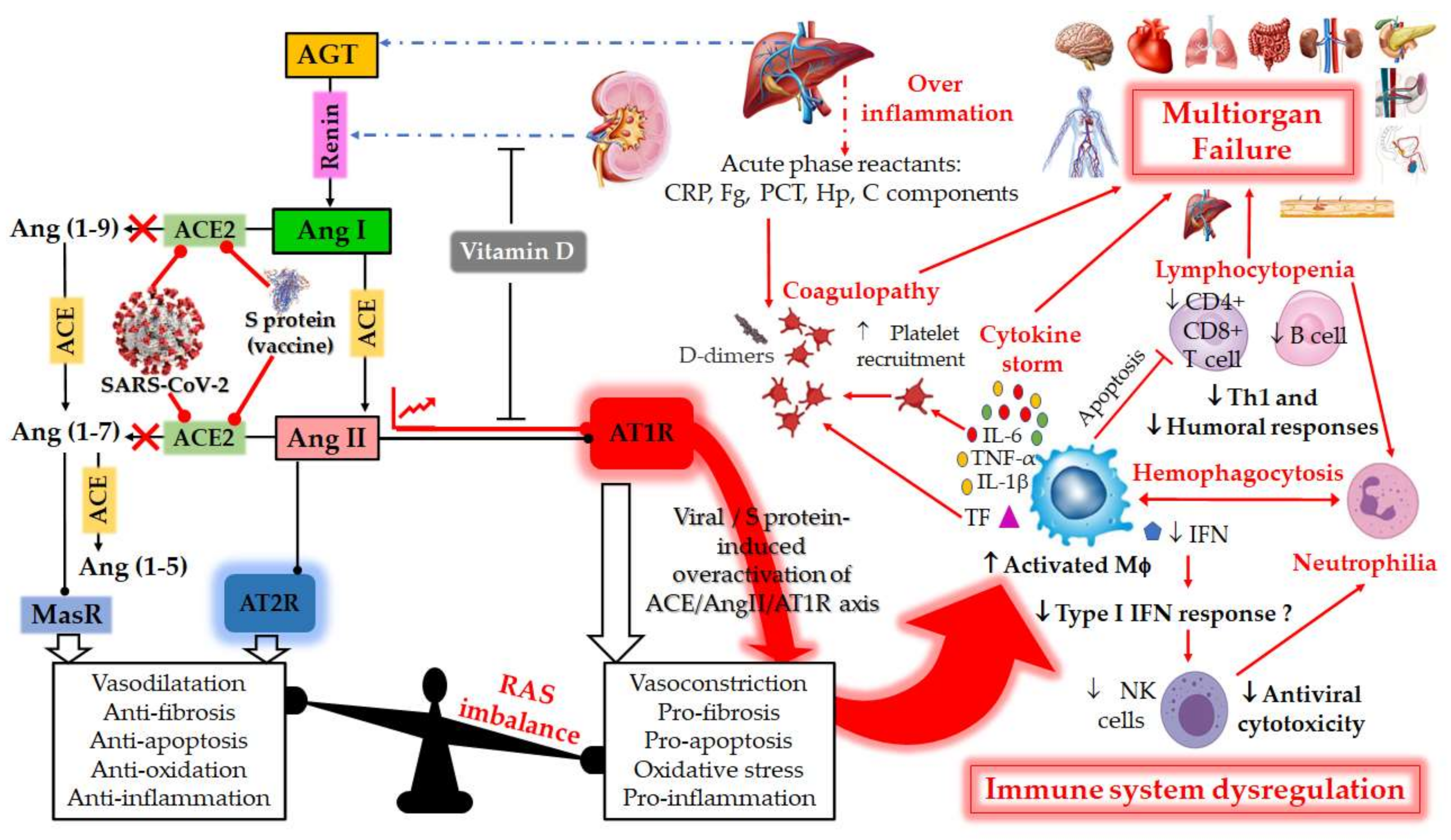 Figure 1. Schematic diagram of the dysregulation in the Renin–Angiotensin System (RAS) and the host immune system caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or by vaccination with mRNA encoding SARS-CoV-2 spike (S) glycoprotein. RAS is a metabolic cascade which supports a series of enzymatic reactions in which the liver secreted AGT is transformed into Ang I by renin, which is a protease secreted by juxtaglomerular kidney cells in response to decrease in blood pressure or sodium load in the distal convoluted tubule. Ang I is subsequently converted to Ang II by ACE which can bind to the AT1R to exert several actions, such as vasoconstriction, pro-fibrosis, pro-apoptosis, oxidative stress and pro-inflammation. ACE2 counterbalances Ang II/AT1R effects by cleaving Ang I and Ang II into Ang-(1–9) and Ang-(1–7), respectively. Ang-(1–9) is also converted into Ang-(1–7), a negative regulator of the RAS, which binds to the MAS receptor to exert protective actions of vasodilatation, anti-fibrosis, anti-apoptosis, anti-oxidative and anti-inflammation. Ang-II can also bind to AT2R to counteract the aforementioned effects mediated by AT1R. The balance between the Ang II/AT1R axis and the ACE2/Ang (1–7)/MasR axis is therefore maintained under physiological conditions. However, during SARS-CoV-2 infection or upon receiving a spike protein-based vaccine, the viral Spike (S) glycoprotein binding to ACE2 receptor induces overactivation of the ACE/Ang II/AT1R axis. This event prevents normal Ang II degradation, the excess of which leads to AT1R overactivation and RAS system imbalance. Such an imbalance is very deleterious for the human body, mainly due to the important immunomodulatory roles of ACE2, which can directly interact with macrophages in the setting of vascular and lung inflammation. Patients with severe COVID-19 infections show hallmarks of sepsis, widely explained by an exacerbation of macrophage activation, including excessive inflammation with the presence of acute phase reactants (such as D-dimer, CRP, etc.), impending cytokine storms and overexpression of IL-1β, IL-2, IL-6, and TNF-α in the early phase of the disease. These induce the production of a compelling number of factors linked to the coagulation cascade (TF, Fb, etc.) and resulting in the onset of thrombi and associated disseminated intravascular coagulation (DIC). The inflammatory response to SARS-CoV-2 also consists of lymphopenia occurring early in >80% of patients and is prognostic, manifested as reduction in—and functional exhaustion of—CD4+ more than CD8+ T cells. Such impaired T cell responses can result from deficient IFN production, as IFNs act on the antigen-presenting cells, T cells, and induce other cytokines and chemokines that regulate T-cell responses. These events lead to imbalance of the innate/acquired immune response, delayed viral clearance and unusual predominance of hyperstimulated macrophage and neutrophil in targeted injured tissues. The permanent immune activation in predisposed elderly adults and patients with cardiovascular risk can lead to hemophagocytosis-like syndrome, with uncontrolled amplification of cytokine production, leading to endothelial dysfunction, tissue damage and multiorgan failure, which is the starting point of a progression towards the serious and fatal complications of COVID-19. This syndrome results from the ineffective activation of cytotoxic CD8+ T lymphocytes and Natural Killer T lymphocytes, and leads to ineffective viral cytotoxicity and weak antibody production. NK cells are regulated by IFNs during coronavirus infection, and patients with severe COVID-19 showed profound depletion and functional exhaustion of NK cells, the dysfunction of which could be due to dysregulation of IFN responses. On the other side, Vitamin D could help avoid the potential deleterious COVID-19 effects sometimes observed following vaccination, by either inhibiting renin secretion or suppressing AT1R overactivation. AGT: angiotensinogen; Ang I: angiotensin I; ACE: angiotensin-converting enzyme; Ang II: angiotensin II; ACE-2: angiotensin-converting enzyme-2; Ang 1–7: angiotensin 1–7; AT1R: angiotensin II type 1 receptor; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; COVID-19: coronavirus disease 2019; CRP, c-reactive protein; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α. Tumor Necrosis Factor-alpha; INF, Interferon; Fg, fibrinogen; PCT, procalcitonin; Hp, haptoglobin; C, complement; MΦ, macrophage; NK, Natural Killer; Th1, T helper type 1; TF, tissue factor.
Figure 1. Schematic diagram of the dysregulation in the Renin–Angiotensin System (RAS) and the host immune system caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or by vaccination with mRNA encoding SARS-CoV-2 spike (S) glycoprotein. RAS is a metabolic cascade which supports a series of enzymatic reactions in which the liver secreted AGT is transformed into Ang I by renin, which is a protease secreted by juxtaglomerular kidney cells in response to decrease in blood pressure or sodium load in the distal convoluted tubule. Ang I is subsequently converted to Ang II by ACE which can bind to the AT1R to exert several actions, such as vasoconstriction, pro-fibrosis, pro-apoptosis, oxidative stress and pro-inflammation. ACE2 counterbalances Ang II/AT1R effects by cleaving Ang I and Ang II into Ang-(1–9) and Ang-(1–7), respectively. Ang-(1–9) is also converted into Ang-(1–7), a negative regulator of the RAS, which binds to the MAS receptor to exert protective actions of vasodilatation, anti-fibrosis, anti-apoptosis, anti-oxidative and anti-inflammation. Ang-II can also bind to AT2R to counteract the aforementioned effects mediated by AT1R. The balance between the Ang II/AT1R axis and the ACE2/Ang (1–7)/MasR axis is therefore maintained under physiological conditions. However, during SARS-CoV-2 infection or upon receiving a spike protein-based vaccine, the viral Spike (S) glycoprotein binding to ACE2 receptor induces overactivation of the ACE/Ang II/AT1R axis. This event prevents normal Ang II degradation, the excess of which leads to AT1R overactivation and RAS system imbalance. Such an imbalance is very deleterious for the human body, mainly due to the important immunomodulatory roles of ACE2, which can directly interact with macrophages in the setting of vascular and lung inflammation. Patients with severe COVID-19 infections show hallmarks of sepsis, widely explained by an exacerbation of macrophage activation, including excessive inflammation with the presence of acute phase reactants (such as D-dimer, CRP, etc.), impending cytokine storms and overexpression of IL-1β, IL-2, IL-6, and TNF-α in the early phase of the disease. These induce the production of a compelling number of factors linked to the coagulation cascade (TF, Fb, etc.) and resulting in the onset of thrombi and associated disseminated intravascular coagulation (DIC). The inflammatory response to SARS-CoV-2 also consists of lymphopenia occurring early in >80% of patients and is prognostic, manifested as reduction in—and functional exhaustion of—CD4+ more than CD8+ T cells. Such impaired T cell responses can result from deficient IFN production, as IFNs act on the antigen-presenting cells, T cells, and induce other cytokines and chemokines that regulate T-cell responses. These events lead to imbalance of the innate/acquired immune response, delayed viral clearance and unusual predominance of hyperstimulated macrophage and neutrophil in targeted injured tissues. The permanent immune activation in predisposed elderly adults and patients with cardiovascular risk can lead to hemophagocytosis-like syndrome, with uncontrolled amplification of cytokine production, leading to endothelial dysfunction, tissue damage and multiorgan failure, which is the starting point of a progression towards the serious and fatal complications of COVID-19. This syndrome results from the ineffective activation of cytotoxic CD8+ T lymphocytes and Natural Killer T lymphocytes, and leads to ineffective viral cytotoxicity and weak antibody production. NK cells are regulated by IFNs during coronavirus infection, and patients with severe COVID-19 showed profound depletion and functional exhaustion of NK cells, the dysfunction of which could be due to dysregulation of IFN responses. On the other side, Vitamin D could help avoid the potential deleterious COVID-19 effects sometimes observed following vaccination, by either inhibiting renin secretion or suppressing AT1R overactivation. AGT: angiotensinogen; Ang I: angiotensin I; ACE: angiotensin-converting enzyme; Ang II: angiotensin II; ACE-2: angiotensin-converting enzyme-2; Ang 1–7: angiotensin 1–7; AT1R: angiotensin II type 1 receptor; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; COVID-19: coronavirus disease 2019; CRP, c-reactive protein; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α. Tumor Necrosis Factor-alpha; INF, Interferon; Fg, fibrinogen; PCT, procalcitonin; Hp, haptoglobin; C, complement; MΦ, macrophage; NK, Natural Killer; Th1, T helper type 1; TF, tissue factor.2. RAS and COVID-19
2.1. RAS: A Portal Entry for SARS-CoV-2
2.2. RAS Imbalance and Underlying Pathologies
Since SARS-CoV-2 uses ACE2 as a key receptor for cellular entry, the latter subsequent downregulation will decrease Ang II degradation, leading therefore to its accumulation [17][33][17,33]. This contributes to a potential dysregulation and overactivation of RAS (Figure 1) [17][36][17,47]. Studies have shown that RAS imbalance worsens COVID-19 prognosis and aggravates its pathogenesis [17][37][17,46]. Therefore, the most common underlying pathophysiology of COVID-19 is a viral acute respiratory distress syndrome (ARDS) coupled with the cytokine storm syndrome [17]. According to current knowledge, the main cause of death in COVID-19 patients in intensive care unit (ICU) is the ARDS secondary to SARS-CoV-2 pneumonia [35]. ARDS is characterized by a hypoxemia with increased capillary–alveolar permeability, reduced lung compliance, alveolar epithelial cell loss, neutrophil infiltration and a diffuse bilateral pulmonary infiltrate that could lead to alveolar and interstitial remodeling and fibrosis [38][48]. This could lead to the loss of pulmonary perfusion regulation and hypoxic vasoconstriction, as well as a low ventilation perfusion ratio [2], thus requiring mechanical ventilation [38][48]. According to Richardson et al., 80% of patients who required mechanical ventilation after COVID-19 infection evolved to death, emphasizing that ARDS is an underlying pathophysiology in COVID-19 patients which may be responsible for the high mortality rates [39][49]. It has been shown that RAS imbalance may influence the pathogenesis of ARDS through Ang II and bradykinin [38][48]. Experiments have revealed that rats with knock-out ACE2 exposed to non-SARS lung damage (such as endotoxin) developed a severe ARDS compared to the wild type rats [40][50]. Therefore, ACE2 was shown to have a protective effect in rat models of acute lung injury, with ACE, Ang II and AT1R being considered as lung injury-promoting elements [40][50]. Imai et al., have shown an upregulation of Ang II by ACE in the pathogenesis of acute lung injury through the AT1a receptor, leading therefore to severe lung failure [28]. A study conducted by Kuba et al., showed that the blockage of ACE2 or its genetic manipulation leads to increased lung edema, vascular permeability and neutrophil accumulation [40][50].2.3. RAS Component Polymorphism
Many studies described the association of the RAS component genetic variation with the prevalence of COVID-19 diseases [36][41][47,68]. Therefore, it has been thought that genetic factors may render the host resistant or susceptible to infection with SARS-CoV-2 [36][47]. The prevalence and disease outcome were linked to ACE polymorphisms [42][36]. The genetic polymorphisms of ACE1 and ACE2 genes may change their levels of expression, therefore leading to an increase in capillary permeability, coagulation, fibrosis and apoptosis in alveolar cells [43][69]. In a pilot study conducted by Cafiero et al., it was found that some genetic variants in the RAS pathway may be potential actors for determining the clinical outcome and the pathological conditions associated COVID-19, such as DIC, interstitial pneumonia, thrombosis, conjunctivitis and the cytokine storm [35]. Thus, inflammation and lung injury caused by ACE2 decrease, following viral binding, could be negatively affected by ACE’s different genotypes, which in turn could increase ACE expression levels and then those of Ang II [42][36]. The knowledge of these polymorphisms could help the management of COVID-19 infected patients [42][36]. The major RAS component polymorphisms are illustrated in Table 1.| RAS Component Gene |
Chromosomal Location | Associated Disease/Phenotype |
Mutations, Polymorphisms and rs Number |
Allele/Genotype Frequencies in Populations and Ethnicities |
References | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACE1 | 17q23.3 | CVD Kidney disease Autoimmune diseases Hypertension Hypercoagulability ARDS Type 2 diabetes Risk of obesity |
Insertion/Deletion (I/D) of a 287-bp | Alu | repeat in intron 16 (rs1799752) |
Lebanese | I | : 0.27, | D | : 0.73 | [44] | [77] | ||||||||||||||||||||
| Indians | I | : 0.55, | D | : 0.45 | Whites | I | : 0.5, | D | : 0.5 | African Americans | I | : 0.41, | D | : 0.59 | [45] | [76] | ||||||||||||||||
| British | I | : 0.31, | D | : 0.69 (ARDS) | I | : 0.49, | D | : 0.51 (healthy population) | [46] | [73] |
||||||||||||||||||||||
| Chinese | I | : 0.705, | D | : 0.295 | [47] | [75] | ||||||||||||||||||||||||||
| Italians | I | : 0.342, | D | : 0.658 | [48] | [56] | ||||||||||||||||||||||||||
| Italians | I | : 0.27, | D | : 0.73 | [35] | |||||||||||||||||||||||||||
| Germans | I | / | I | : 0.27, | I | / | D | : 0.43, | D | / | D | : 30 | [38] | [48] | ||||||||||||||||||
| Indians | I | : 0.575, | D | : 0.425 | [49] | [78] | ||||||||||||||||||||||||||
| ACE2 | Xp22.2 | Cardiovascular risk, Retinopathy in type-2 Diabetes Mellitus, Hypertension and Hypertensive left ventricular hypertrophy | c.*1860-449C > T SNP (rs2074192) |
Italians | C | : 0.56, | T | : 0.44 | [35] | |||||||||||||||||||||||
| c.*264+788T > C (rs2106809) |
Italians | A | : 0.77, | G | : 0.33 | |||||||||||||||||||||||||||
| c.2115-268A > T SNP (rs233574) |
Africans | C | : 0.92, | T | : 0.08 | Europeans | C | : 0.67, | T | : 0.33 | East Asians | C | : 0.996, | T | : 0.004 | South Asians | C | : 0.814, | T | : 0.814 | Americans | C | : 0.767, | T | : 0.233 | [50] | [79] | |||||
| c.1402A > G p.Ile468Val SNP (rs191860450) |
East Asians | with an allele frequency (AF) = 0.011 | [16] | |||||||||||||||||||||||||||||
| c.1022A > G p.Lys341Arg SNP (rs138390800) |
Africans | AF = 4 × 10 | −3 | |||||||||||||||||||||||||||||
| c.2191C > T p.Leu731Phe SNP (rs147311723) |
Africans | AF = 0.014 |
||||||||||||||||||||||||||||||
| c.631G > A p.Gly211Arg SNP (rs148771870) |
Europeans | AF = 2 × 10 | −3 | South Asians | AF = 1.9 × 10 | −3 | ||||||||||||||||||||||||||
| c.2089A > G p.Arg697Gly SNP (rs751603885) |
South Asians | AF = 2.4 × 10 | −3 | |||||||||||||||||||||||||||||
| c.2074T > C p.Ser692Pro SNP (rs14903946) |
Africans | AF = 6 × 10 | −3 | |||||||||||||||||||||||||||||
| c.55T > C p.Ser19Pro SNP (rs73635825) |
Africans | AF = 3 × 10 | −3 | |||||||||||||||||||||||||||||
| AGT | 1q42.2 1q42–43 |
Hypertension Heart failure Myocardial infraction |
c.704T > C p.Met235Thr (aka Met268Thr) SNP (rs699) |
Tunisians | M | / | M | : 0.291, | M | / | T | : 0.291 | T | / | T | :0.419 | [51] | [80] | ||||||||||||||
| Vietnamese | T | : 0.92, | M | : 0.08 | [52] | [81] | ||||||||||||||||||||||||||
| Iranians | T | : 0.39, | M | : 0.61 | [53] | [82] | ||||||||||||||||||||||||||
| Indians | M | : 0.52, | D | : 0.48 | [49] | [78] | ||||||||||||||||||||||||||
| New Zealanders | T | / | T | : 0.19, | T | / | M | : 0.47, | M | / | M | : 0.34 | [54] | [83] | ||||||||||||||||||
| c.521C > T p.Thr174Met SNP (rs4762) |
New Zealanders | T | / | T | : 0.7, | T | / | M | : 0.2, | M | / | M | : 0.1 | [54] | [83] | |||||||||||||||||
| AT1R | 3q21–q25 | Systolic blood pressure Left ventricular hypertrophy Hypertension Aortic stiffness Myocardial infarction Carotid intimal-medial thickening, CAD and stroke, Overweight, Diabetes |
c.1166A > C in the 3′ UTR SNP (rs5186) |
Egyptians | C | :0.24, | A | :0.76 (control group) | C | :0.34, | G | :0.66 (premature CAD patients) | [55] | [84] | ||||||||||||||||||
| Jordanians | Higher frequency of | A | allele | [56] | [85] | |||||||||||||||||||||||||||
| Iranians | Higher frequency of | A | allele | [57] | [86] | |||||||||||||||||||||||||||
| AT2R | Xq23–26 | Metabolic syndrome | −1332A > G SNP (rs1403543) |
Egyptians | A | :0.55, | G | :0.45 (control group) | A | :0.41, | G | :0.50 (premature CAD patients) | [55] | [84] |
3. Correlation of Habits, Gender and COVID-19 with RAS Polymorphism
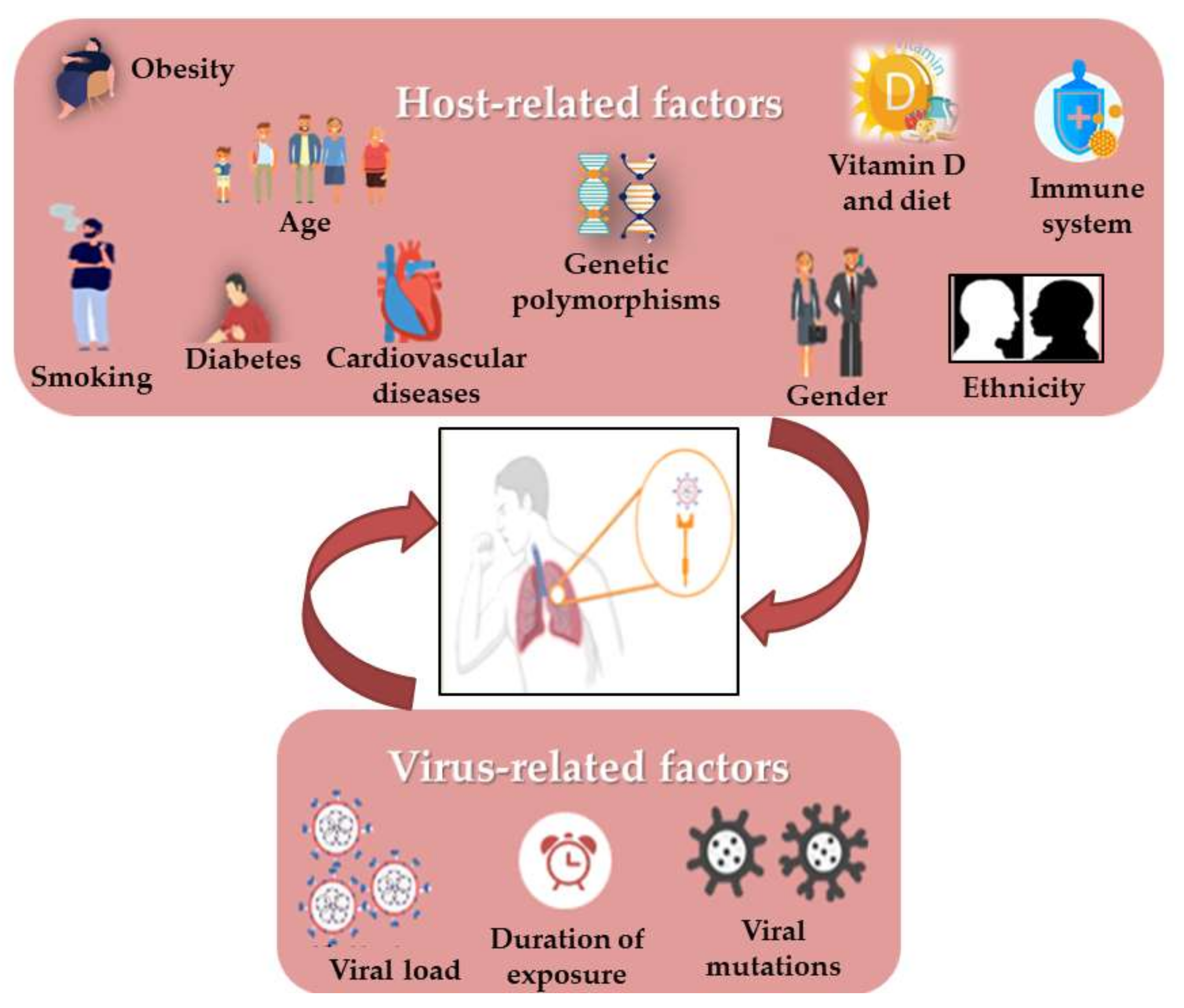
Still now, it is surprising that some COVID-19 patients are asymptomatic while others have much more severe disease outcomes. Moreover, viral respiratory infections are generally more harmful in children than in adults, but this appeared to be inverted in SARS-CoV-2 infection. It has been shown that some virus-related factors (viral load in the inoculum, the exposure duration and viral genomic mutations) can influence the severity and outcome of the disease [58][100] (Figure 2). Similarly, it quickly became obvious that several risk factors such as age, gender, the presence of comorbidities (such as smoking, immune status, diabetes, cardiovascular disease including hypertension, respiratory diseases and cancer) and the genetic background seem to control the manifestations and outcome of infection [59][60][61][62][41,45,101,102]. In addition, it has also drawn attention to vitamin D deficiency [63][103], as well as the ethnic differences, such as the black and South Asian ethnicities, and the lower socioeconomic statuses which are considered to increase risks [64][54]. Most mortalities occurred in the elderly and in men compared to women (4.7% vs. 2.8%). Moreover, the death of patients with no pre-existing conditions is approximately 10 times lower than in those with pre-existing ones. As in the 2003 SARS epidemic, hypertension has the most comorbidity frequency in non-survivors, in addition to cardiovascular diseases, obesity and diabetes, especially in smokers [64][65][54,90]. There is evidence that the RAS upregulation in the adipose tissue may lead to hypertension and insulin resistance in obese people. [66][37]