Nanocelluloses are promising bio-nano-materials for use as water treatment materials in environmental protection and remediation. This review aims at giving an overview of nanocellulose requirements concerning emerging nanotechnologies of waster treatments and purification, i.e., adsorption, absorption, flocculation, photocatalytic degradation, disinfection, antifouling, ultrafiltration, nanofiltration, and reverse osmosis.
- nanoparticles
- nanocrystals
- nanowhiskers
- nanofibers
1. Introduction
2. Market, Production, and Challenges of Nanocelluloses
The industrial-scale production and market of nanocellulose are rapidly expanding worldwide as they are environmentally friendly, non-toxic, sustainable, low-cost, and highly efficient materials for a wide variety of applications, including water treatment. At present, different forms of nanocellulose are available on the market, which is forecasted to achieve USD 783 Million by 2025 according to Markets and Markets. The global market size of nanocelluloses is expected to grow at 21.4% of a compound annual growth rate from 2020 to 2026 [4]. To date, researchers have developed several nanofabrication techniques for the production of nanocelluloses: (1) Mechanical methods such as grinding, homogenization, refining, aqueous counter collision, cryo-crushing are normally used to disrupt the cellulose microfibers from plant materials down to submicron or nanoscale dimensions [10]. (2) physical techniques such as steam explosion, wet spinning, dry spinning, melt spinning, or electrospinning have been used to produce electrospun cellulose acetate nanofiber (100 to 1000 nm in width and over 1 μm in length) [10][11]. (3) Chemical treatment of lignocellulose materials including alkali treatment, acid hydrolysis, sulfonation, and ionic liquid approaches with selected chemicals used to provide nanocelluloses with specific functionalities and high purity [12][13][14]. (4) biological treatment such as enzymatic hydrolysis has been applied before mechanical cellulose fragmentation to reduce chemical waste and energy consumption [4][15]. The surface modifications have been successfully used to tune nanocellulose reactivity towards different water pollutant types (e.g., biological species, inorganic pollutants, heavy metals). The modification processes include and are not limited to: (1) carboxylic acid halides create ester linkages, (2) sulfuric acid treatment provides sulfate esters, (3) epoxides create ether linkages, (4) acid anhydrides create ester linkages, (5) TEMPO mediated hypochlorite oxidation creates carboxylic acids, (6) isocyanates create urethane linkages, (7) chlorosilanes create an oligomeric silylated layer; (8) halogenated acetic acids create carboxymethyl surfaces [16]. The chemical surface modifications modulate the nanocellulose hydrophobic and hydrophilic properties because its intrinsic hydrophilicity contributes to the swelling and loss of mechanical strength or stiffness of the filter material. High hydrophobicity might increase the flow resistance and/or modify the selectivity for the adsorbed species. However, certain current drawbacks hinder the further expansion of the nanocellulose utilization as mainly related to their processing conditions: (1) High dispersion stability of individual nanocellulose particles makes their separation from the water system difficult and necessitates the addition of salt or pH alteration to recover them after the water treatment process; (2) Dispersion of nanocellulose in hydrophobic polymer matrices (membranes) remains a critical issue. However, the dispersion of nanocelluloses in polymer blends for sustainable wastewater treatment applications can be achieved by surface grafting of nanocelluloses with low molecular weight polymers; and (3) High water and energy consumption and yield are the main challenges in the preparation process, along with by-product toxicity [4]. For example, acid wastewater is typically generated from the washing process for neutralizing the pH value of the nanocellulose suspension.3. Adsorbents for Hazardous Metal Removal
Adsorption of hazardous (radioactive and heavy) metal ions is considered as one of the suitable water treatment methods due to due to its high efficiency, low cost, and ease of operation. Numerous studies reported that the nanosorbents remove radioactive and heavy metals from wastewater, e.g., carbon tube, graphene oxide, polymeric, zeolites, metal and metal oxides nanosorbents [17]. For using nanocellulose-based adsorbents, ion exchange and chemical-complexation are the main two mechanisms concerned for the uptake of heavy metals. The ion-exchange mechanism involves the adsorption of hazardous metal ions (Mn+) takes the place of other ions (K+, Na+, H+) already associated with the nanocellulose surface. In chemical complexation, the carboxyl (-COO−) and hydroxyl (-OH) groups of the nanocelluloses have specific site interactions with particular hazardous metal ions (Mn+). The maximum adsorption capacity of nanocelluloses is limited by their surface area, functionality, and stoichiometry rules which cannot exceed half the content of surface ionic sites.
4. Adsorbents for Hazardous Organic Pollutants Removal
The affinity of native cellulose microfibers towards organic pollutants is 100 to 500 times lower than that of conventional nanomaterials, such as zeolite or activated carbon, due to the low number of active sites for interaction with the organic pollutants [18]. Alternatively, surface-modified nanocelluloses have been tested as support materials for the adsorption of various organic pollutants [19]. This is mainly explained by their robust mechanical properties, the high specific surface area that allows creating active interaction sites after functionalization, and the small pore size of their filters/membranes. As the nanocellulose intrinsic hydrophilicity is not suitable for the adsorption of organic molecules, surface modifications, and/or formation of nanocomposite materials (e.g., porous films or aerogels with controllable porosity) are required to improve the adsorption and filtration capacity.
5. Flocculants and Coagulants for Suspended Materials
Flocculants are agents that can promote flocculation of colloids and suspended particles in liquids to aggregate them for floc formation [20][21]. Nanocelluloses such as CNF and CNC their modified counterparts have been investigated as flocculants for the treatment and elimination of contaminants from wastewater. These nanoflocculants induce flocculation of the suspended particles in contaminated wastewater by neutralizing the surface charge of particles or by forming bridges between the individual suspended particles. Figure 8 explains how anionic nanocelluloses (CNFs and CNCs) function as flocculants for the capture and removal of charged pollutants [22]. In contrast to native cellulose, there are several characteristic features of nanocelluloses (CNFs and CNCs) make them an ideal flocculants candidate for water treatment: (1) small size and high-surface-area rod-like morphology that give rise to percolation at low concentrations; (2) CNFs and CNCs can improve the formation of flocs compared to native fibers. In comparison between CNFs and CNCs, the higher electrostatic repulsion and rigidity of CNCs than CNFs will prevent the occurrence of physical and chemical entanglements contributing to reduced risk of gelation.6. Wettable Materials for Oil/Water Separation
Efficient water/oil separation is accomplished by separating water and oil, followed by or in combination with oil adsorption. Currently, the most common water/oil separation methods (sedimentation, centrifugation, or hydrocyclone) are based on differences in gravity or density [23][24]. Recently, nanocelluloses (CNFs and CNCs) incorporation in membranes for oil separation offers interesting opportunities, together with the (partial) modification of the original hydrophilic surface properties towards higher hydrophobicity and/or balanced amphiphilicity [25]. Besides CNFs and CNCs, electrospun cellulose nanoyarn membranes with grafted hydrophobic moieties have been used for oil removal. The step-by-step fabrication of oil/water separation membranes by electrospinning of cellulose acetate, followed by their deacetylation [23]. Interestingly, electrospun cellulose acetate nanofibers are super-amphiphilic in air, oleophobic in water, and super-hydrophilic in oil. The membranes are characterized by high separation flux (up to 38,000 L/m2·h) and gravity-based separation efficiency (up to 99.97%) for chloroform/water mixtures [23]. Similarly, ultralight carbon aerogels prepared from hydrophobized cellulose microfibers can be valuable for selective oil sorption [26]. These sponges show 99% of porosity, 0.01 g/cm3 density, good hydrophobicity, reusability, and very high adsorption capacity (>86 g/g) towards paraffin oil. Surface hydrophobization of BNC membranes has been modified with trimethylchlorosilane for the efficient removal of plant oil from water [27], by dipping BNC aerogels into liquid phase trimethylchlorosilane followed by freeze-drying (Figure 1). The obtained very hydrophobic (water/air contact angle as high as 146.5°) and highly porous (≈ 99.6%) surface-modified BNCs offer high selectivity for oil adsorption from water, with absorption capacities up to 185 g/g [27]. Incorporation of magnetic nanoparticles onto nanocelluloses via blending or in situ hydrolysis of metal precursors that have been used for oil removal from wastewater. Magnetic nanocellulose aerobeads, fabricated by freeze-drying of iron oxide (Fe3O4)-containing spherical CNFs (from used cardboard boxes), also show very good selectivity towards oil removal. These aerobeads (0.005 g/cm3 of density and 99% of porosity) possess excellent absorption efficiency towards various oils and organic solvents, especially castor oil (279 g/g).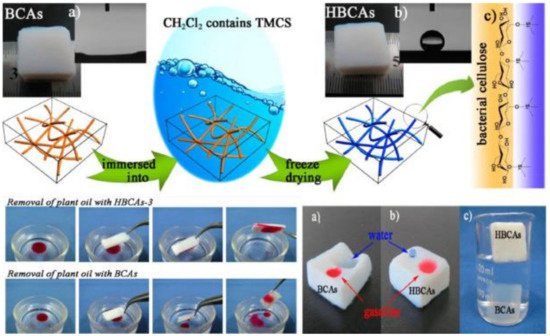
7. Photocatalytic Materials for Hazardous Pollutants Degradation
Photocatalysis has emerged as an environmentally friendly, low-cost, and handy approach for the degradation of organic pollutants and/or dyes in wastewater. Even though nanocelluloses alone exhibits limited photocatalytic activity under the visible light-UV region, conventional metal oxide photocatalyst (ZnO [28] and TiO2 [29]) have been added to enhance the photocatalytic activity. These photocatalytic materials (individual particles, thin-film, membranes) have been developed using cellulose-based metal oxide nanostructures in the form of under UV and visible light irradiation. Photocatalysis harnesses photon energy to produce active radicals that promote the decomposition of organic pollutants [30]. When the photon energy is above the semiconductor energy gap, semiconductor materials can generate active hydroxyl radicals, leading to the excitation of electrons from the valence band into the conduction region [31]. This creates a hole that can generate hydroxyl radicals in an alkaline medium [32]. These radicals are highly reactive and can enhance the oxidation of recalcitrant chemicals in wastewater, leading to complete degradation of organic pollutants into nontoxic byproducts (i.e., CO2 and H2O) [33]. Active hydroxyl free radical radicals attack organic materials via four known pathways: radical addition, hydrogen abstraction, electron transfer, and radical combination [34]. The main challenge associated with semiconductor NP use in photocatalysis is their removal from the liquid after the reaction. Poor visible light absorption nanocellulose/TiO2 and nanocellulose/ZnO is the limitation of such photocatalysts due to the wide bandgap. Similarly, separation of these nanocomposites is difficult from reaction mixture due to non-magnetic characteristics.8. Membrane Materials for Wastewater Treatment
Membrane technology has emerged as a favorite processing and separation method (filtration, extraction, and distillation) for reclaiming water from salty and contaminated water streams for their re-use by humans. The use of nanocellulose based membranes for water purification and/or desalination is a highly efficient and environmentally responsible method because of the low energy consumption and high selectivity. The operational characteristics of nanocellulose based membranes are mostly determined by two parameters: their water permeation flux value, and their specific pollutant removal capacity. The nanocellulose pore size and surface conditions are important parameters for controlling the filter efficiency. The membrane rejection might be explained by two mechanisms: (i) size-exclusion effects, and (ii) the membrane chemical or physical affinity for the pollutant. In the first mechanism (size-exclusion), the pollutant is selectively rejected depending on its size relative to the membrane pore size. The functioning and efficiency of nanocellulose based membranes depend on their selective retention of pollutants. Depending on the pollutant size, cellulose-based membranes are categorized into [35]:- (1) Microfiltration membranes with microspores of 10 µm to 100 nm; to be used for removal of suspended particles and Bacteria (200 nm–30 micron)
- (2) Ultrafiltration membranes with nanopore range from 100 nm to 2 nm; it is suitable for removal of nanoparticles and viruses with sizes 50–200 nm
- (3) Nanofiltration membranes with nanopore size range from 2 nm to 1 nm; to be used for removal of organic pollutants (0.7–1.5 nm)
- (4) Reverse osmosis with sub-nanopore size from 0.1 to 1 nm; to be used for hazardous metal ion (0.1–0.7) removal.
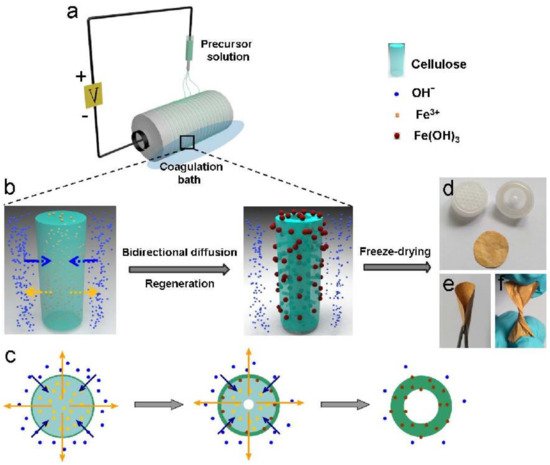
9. Water Disinfection Materials from Pathogenic Microorganisms
Disinfection processes are the last and most important step in the wastewater treatment process, i.e., removal, deactivation or killing of pathogenic microorganisms. Over the past decades, several nanomaterials (metal, metal oxides, and polymer NPs) have been used as disinfectants to reduce the harmful disinfection by-products [55]. Nanocelluloses on their own have negligible effects on microbial inhibition, and they can serve as a carbon source for their development, especially bacteria, algae, and fungi. Therefore, antimicrobial and antibacterial activities should be added to nanocellulose based materials to inhibit microbe’s growth in contaminated water. In this aspect, antibacterial features can be added to nanocelluloses for instance by surface modification with antimicrobial polymers, metal NPs (e.g., Ag, Au), and metal oxide NPs (e.g., TiO2, CuO, ZnO, MgO) [56]. Surface modification of nanocelluloses with oxidized groups gives active sites (TEMPO oxidation) provides nanocelluloses with functionalized carboxyl groups with high affinity towards E. coli (rejection ~96–99%). The introduction of negative surface charges (carboxyl groups) offers resistance against Gram-positive and Gram-negative bacteria, and a high binding affinity to the negative charge near the bacterial membrane can be provided. The carboxyl groups on the nanocelluloses surface do not intrinsically act as antibacterial agents, but they can be loaded with appropriate antibacterial compounds. For instance, cationic surface modification by quaternary ammonium compounds, such as ammonium bromides [57], and ammonium chlorides [58], is a straightforward method to introduce the antibacterial activity.10. Current Challenges and Limitations
Even though cellulose-based composite materials show promising application in water purification for the elimination of heavy metal ions and organic dyes, it remains a big challenge to control the main factors of porosity, permeability, and specific adsorption. The control over the morphology of the membranes can be controlled through the presented methods in this review (e.g., electrospinning, coating, casting, freeze-drying), but a continuous and most cost-efficient industrial process for fabrication of the membranes still follows an easy papermaking-like route. However, urgent limitations to be resolved are attributed to the energetic efficiency for the formation of nanocellulose membranes (e.g., fabrication of nanofibrils, dewatering of the fiber mats), quality of the produced membranes (e.g., the occurrence of defects in the membranes and homogeneity in pore sizes), and performance (e.g., the relatively thick membranes are required to compensate for defects while decreasing the permeability and increasing pressure drops). In particular, the economic and cost-efficient upscaling of production of suitable membranes needs to be developed, in particular, controlling film formation and drying procedures to improve the morphology of high-flux membranes. The strong tendency for agglomeration of nanocellulose during film drying can be resolved by the deposition of nanocellulose onto given filters or membrane structures providing them with a thin functional layer.11. Conclusions
Developing eco-friendly water treatment materials with improved performance has received significant academic and industrial attention. To date, a large variety of approaches have been developed to address arguably one of the key challenges for humanity in the 21st century: clean water. Among the different nanomaterials, nanocelluloses are promising sustainable materials for wastewater treatment, in terms of performance and efficiency, and also lightweight with strong mechanical strength, chemical inertness, and versatile surface chemistry, inexpensive production costs, and safe handling compared with inorganic nanomaterials. The improvement of nanocellulose production has led many researchers to fabricate nanocellulose-based materials (individual NPs, hydrogels, aerogels, sponges, membranes) for wastewater treatment. Different nanocellulose forms (cellulose nanocrystals, cellulose fibers, bacterial cellulose) have been successfully investigated for water treatment, particularly in membranes and filters (size exclusion, e.g., for nanoparticle filtration, or affinity membranes) and also as adsorbents (e.g., heavy metal ions, dyes, drugs, pesticides, fertilizers).References
- Ranade, V.V.; Bhandari, V.M. Industrial Wastewater Treatment, Recycling, and Reuse: An Overview. In Industrial Wastewater Treatment, Recycling and Reuse; Butterworth-Heinemann: Oxford, UK, 2014; pp. 1–80. ISBN 9780444634030. Barhoum, A.; Jeevanandam, J.; Rastogi, A.; Samyn, P.; Boluk, Y.; Dufresne, A.; Danquah, M.K.; Bechelany, M. Plant celluloses, hemicelluloses, lignins, and volatile oils for the synthesis of nanoparticles and nanostructured materials. Nanoscale 2020, 12, 22845–22890.
- Wiesmann, U.; Choi, I.S.; Dombrowski, E.M. Fundamentals of Biological Wastewater Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 9783527312191.
- Barhoum, A.; Jeevanandam, J.; Rastogi, A.; Samyn, P.; Boluk, Y.; Dufresne, A.; Danquah, M.K.; Bechelany, M. Plant celluloses, hemicelluloses, lignins, and volatile oils for the synthesis of nanoparticles and nanostructured materials. Nanoscale 2020, 12, 22845–22890.
- Barhoum, A.; Li, H.; Chen, M.; Cheng, L.; Yang, W.; Dufresne, A. Emerging Applications of Cellulose Nanofibers. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2019; pp. 1131–1156.
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392.
- Hassan, E.; Hassan, M.; Abou-Zeid, R.; Berglund, L.; Oksman, K. Use of Bacterial Cellulose and Crosslinked Cellulose Nanofibers Membranes for Removal of Oil from Oil-in-Water Emulsions. Polymers 2017, 9, 388.
- Nnaji, C.O.; Jeevanandam, J.; Chan, Y.S.; Danquah, M.K.; Pan, S.; Barhoum, A. Engineered nanomaterials for wastewater treatment: Current and future trends. In Fundamentals of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 129–168.
- Ahankari, S.; George, T.; Subhedar, A.; Kar, K.K. Nanocellulose as a sustainable material for water purification. SPE Polym. 2020, 1, 69–80.
- Hassan, M.L.; Fadel, S.M.; Abouzeid, R.E.; Elseoud, W.S.A.; Hassan, E.A.; Berglund, L.; Oksman, K. Water purification ultrafiltration membranes using nanofibers from unbleached and bleached rice straw. Sci. Rep. 2020, 10, 11278.
- Zhang, K.; Barhoum, A.; Xiaoqing, C.; Li, H.; Samyn, P. Cellulose Nanofibers: Fabrication and Surface Functionalization Techniques. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2019; pp. 409–449.
- Li, R.; Zhang, L.; Wang, P. Rational design of nanomaterials for water treatment. Nanoscale 2015, 7, 17167–17194.
- Shak, K.P.Y.; Pang, Y.L.; Mah, S.K. Nanocellulose: Recent advances and its prospects in environmental remediation. Beilstein J. Nanotechnol. 2018, 9, 2479–2498.
- El-Gendy, A.; Abou-Zeid, R.E.; Salama, A.; Diab, M.; El-Sakhawy, M. TEMPO-oxidized cellulose nanofibers/polylactic acid/TiO2 as antibacterial bionanocomposite for active packaging. Egypt. J. Chem. 2017, 60, 1007–1014.
- Abouzeid, R.E.; Khiari, R.; Salama, A.; Diab, M.; Beneventi, D.; Dufresne, A. In situ mineralization of nano-hydroxyapatite on bifunctional cellulose nanofiber/polyvinyl alcohol/sodium alginate hydrogel using 3D printing. Int. J. Biol. Macromol. 2020, 160, 538–547.
- Hassan, M.; Berglund, L.; Hassan, E.; Abou-Zeid, R.; Oksman, K. Effect of xylanase pretreatment of rice straw unbleached soda and neutral sulfite pulps on isolation of nanofibers and their properties. Cellulose 2018, 25, 2939–2953.
- Patel, D.K.; Dutta, S.D.; Lim, K.-T. Nanocellulose-based polymer hybrids and their emerging applications in biomedical engineering and water purification. RSC Adv. 2019, 9, 19143–19162.
- Xu, Q.; Poggi, G.; Resta, C.; Baglioni, M. Grafted nanocellulose and alkaline nanoparticles for the strengthening and deacidification of cellulosic artworks. J. Colloid Interface Sci. 2020, 576, 147–157.
- El-Sayed, M.E. Nanoadsorbents for water and wastewater remediation. Sci. Total Environ. 2020, 739, 139903.
- Alila, S.; Boufi, S. Removal of organic pollutants from water by modified cellulose fibres. Ind. Crop. Prod. 2009, 30, 93–104.
- Shahnaz, T.; Priyan, V.V.; Pandian, S.; Narayanasamy, S. Use of Nanocellulose extracted from grass for adsorption abatement of Ciprofloxacin and Diclofenac removal with phyto, and fish toxicity studies. Environ. Pollut. 2021, 268, 115494.
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45.
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process. Saf. Environ. Prot. 2014, 92, 489–508.
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197.
- Wang, W.; Lin, J.; Cheng, J.; Cui, Z.; Si, J.; Wang, Q.; Peng, X.; Turng, L.-S. Dual super-amphiphilic modified cellulose acetate nanofiber membranes with highly efficient oil/water separation and excellent antifouling properties. J. Hazard. Mater. 2020, 385, 121582.
- Zhang, Z.; Sèbe, G.; Rentsch, D.; Zimmermann, T.; Tingaut, P. Ultralightweight and Flexible Silylated Nanocellulose Sponges for the Selective Removal of Oil from Water. Chem. Mater. 2014, 26, 2659–2668.
- Sharma, P.R.; Sharma, S.K.; Lindström, T.; Hsiao, B.S. Nanocellulose-Enabled Membranes for Water Purification: Perspectives. Adv. Sustain. Syst. 2020, 4, 1900114.
- Meng, Y.; Young, T.M.; Liu, P.; Contescu, C.I.; Huang, B.; Wang, S. Ultralight carbon aerogel from nanocellulose as a highly selective oil absorption material. Cellulose 2015, 22, 435–447.
- Sai, H.; Fu, R.; Xing, L.; Xiang, J.; Li, Z.; Li, F.; Zhang, T. Surface Modification of Bacterial Cellulose Aerogels’ Web-like Skeleton for Oil/Water Separation. ACS Appl. Mater. Interfaces 2015, 7, 7373–7381.
- Zheng, W.-L.; Hu, W.-L.; Chen, S.-Y.; Zheng, Y.; Zhou, B.-H.; Wang, H.-P. High photocatalytic properties of zinc oxide nanoparticles with amidoximated bacterial cellulose nanofibers as templates. Chin. J. Polym. Sci. 2014, 32, 169–176.
- Wang, S.-D.; Ma, Q.; Liu, H.; Wang, K.; Ling, L.-Z.; Zhang, K.-Q. Robust electrospinning cellulose 2 ultrafine fibers for dyeing water treatment by photocatalytic reactions. RSC Adv. 2015, 5, 40521–40530.
- Rehan, M.; Barhoum, A.; Khattab, T.; Gätjen, L.; Wilken, R. Colored, photocatalytic, antimicrobial and UV-protected viscose fibers decorated with Ag/Ag2CO3 and Ag/Ag3PO4 nanoparticles. Cellulose 2019, 26, 5437–5453.
- Leong, W.S.; Luo, X.; Li, Y.; Khoo, K.H.; Quek, S.Y.; Thong, J.T.L. Low Resistance Metal Contacts to MoS2 Devices with Nickel-Etched-Graphene Electrodes. ACS Nano 2015, 9, 869–877.
- Rehan, M.; Barhoum, A.; Van Assche, G.; Dufresne, A.; Gätjen, L.; Wilken, R. Towards multifunctional cellulosic fabric: UV photo-reduction and in-situ synthesis of silver nanoparticles into cellulose fabrics. Int. J. Biol. Macromol. 2017, 98, 877–886.
- Barhoum, A.; Melcher, J.; Van Assche, G.; Rahier, H.; Bechelany, M.; Fleisch, M.; Bahnemann, D.B.D. Synthesis, growth mechanism, and photocatalytic activity of Zinc oxide nanostructures: Porous microparticles versus nonporous nanoparticles. J. Mater. Sci. 2017, 52, 2746–2762.
- Ren, C.; Yang, B.; Wu, M.; Xu, J.; Fu, Z.; Lv, Y.; Guo, T.; Zhao, Y.; Zhu, C. Synthesis of Ag/ZnO nanorods array with enhanced photocatalytic performance. J. Hazard. Mater. 2010, 182, 123–129.
- Zeng, J.; Liu, S.; Cai, J.; Zhang, L. TiO2 Immobilized in Cellulose Matrix for Photocatalytic Degradation of Phenol under Weak UV Light Irradiation. J. Phys. Chem. C 2010, 114, 7806–7811.
- Patil, K.; Jeong, S.; Lim, H.; Byun, H.-S.; Han, S. Removal of volatile organic compounds from air using activated carbon impregnated cellulose acetate electrospun mats. Environ. Eng. Res. 2019, 24, 600–607.
- Samyn, P.; Barhoum, A. Engineered nanomaterials for papermaking industry. In Fundamentals of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 245–277.
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A.P. Nanocellulose-based materials for water purification. Nanomaterials 2017, 7, 57.
- Tavakolian, M.; Jafari, S.M.; van de Ven, T.G.M. A Review on Surface-Functionalized Cellulosic Nanostructures as Biocompatible Antibacterial Materials. Nano-Micro Lett. 2020, 12, 1–23.
- Zhao, J.; Lu, Z.; He, X.; Zhang, X.; Li, Q.; Xia, T.; Zhang, W.; Lu, C.; Deng, Y. One-Step Fabrication of Fe(OH)3@Cellulose Hollow Nanofibers with Superior Capability for Water Purification. ACS Appl. Mater. Interfaces 2017, 9, 25339–25349.
- Wang, X.; Ding, B.; Sun, G.; Wang, M.; Yu, J. Electro-spinning/netting: A strategy for the fabrication of three-dimensional polymer nano-fiber/nets. Prog. Mater. Sci. 2013, 58, 1173–1243.
- Mansouri, J.; Harrisson, S.; Chen, V. Strategies for controlling biofouling in membrane filtration systems: Challenges and opportunities. J. Mater. Chem. 2010, 20, 4567–4586.
- Kong, L.; Yin, X.; Yuan, X.; Zhang, Y.; Liu, X.; Cheng, L.; Zhang, L. Electromagnetic wave absorption properties of graphene modified with carbon nanotube/poly(dimethyl siloxane) composites. Carbon 2014, 73, 185–193.
- Hassan, M.; Berglund, L.; Abou-Zeid, R.; Hassan, E.; Abou-Elseoud, W.; Oksman, K. Nanocomposite Film Based on Cellulose Acetate and Lignin-Rich Rice Straw Nanofibers. Materials 2019, 12, 595.
- Xu, X.; Zhou, J.; Jiang, L.; Lubineau, G.; Chen, Y.; Wu, X.-F.; Piere, R. Porous core-shell carbon fibers derived from lignin and cellulose nanofibrils. Mater. Lett. 2013, 109, 175–178.
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Highly Permeable Polymer Membranes Containing Directed Channels for Water Purification. ACS Macro Lett. 2012, 1, 723–726.
- Wang, J.-G.; Yang, Y.; Huang, Z.-H.; Kang, F. A high-performance asymmetric supercapacitor based on carbon and carbon–MnO2 nanofiber electrodes. Carbon 2013, 61, 190–199.
- Ferraz, E.R.A.; Oliveira, G.A.R.; Grando, M.D.; Lizier, T.M.; Zanoni, M.V.B.; Oliveira, D.P. Photoelectrocatalysis based on Ti/TiO2 nanotubes removes toxic properties of the azo dyes Disperse Red 1, Disperse Red 13 and Disperse Orange 1 from aqueous chloride samples. J. Environ. Manag. 2013, 124, 108–114.
- Barud, H.S.; Souza, J.L.; Santos, D.B.; Crespi, M.S.; Ribeiro, C.; Messaddeq, Y.; Ribeiro, S. Bacterial cellulose/poly(3-hydroxybutyrate) composite membranes. Carbohydr. Polym. 2011, 83, 1279–1284.
- Asper, M.; Hanrieder, T.; Quellmalz, A.; Mihranyan, A. Removal of xenotropic murine leukemia virus by nanocellulose based filter paper. Biologicals 2015, 43, 452–456.
- Metreveli, G.; Wågberg, L.; Emmoth, E.; Belák, S.; Strømme, M.; Mihranyan, A. A Size-Exclusion Nanocellulose Filter Paper for Virus Removal. Adv. Healthc. Mater. 2014, 3, 1546–1550.
- Quellmalz, A.; Mihranyan, A. Citric Acid Cross-Linked Nanocellulose-Based Paper for Size-Exclusion Nanofiltration. ACS Biomater. Sci. Eng. 2015, 1, 271–276.
- Takai, M.; Nonomura, F.; Inukai, T.; Fujiwara, M.; Hayashi, J. Filtration and permeation characteristics of bacterial cellulose composite. Sen’i Gakkaishi 1991, 47, 119–129.
- Fang, Q.; Zhou, X.; Deng, W.; Zheng, Z.; Liu, Z. Freestanding bacterial cellulose-graphene oxide composite membranes with high mechanical strength for selective ion permeation. Sci. Rep. 2016, 6, 33185.
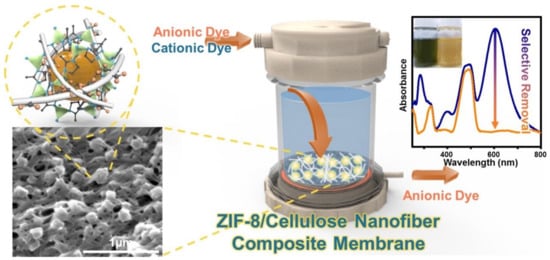
- Song, Y.; Seo, J.Y.; Kim, H.; Beak, K.Y. Structural control of cellulose nanofibrous composite membrane with metal organic framework (ZIF-8) for highly selective removal of cationic dye. Carbohydr. Polym. 2019, 222, 115018.
- Hossain, F.; Perales-Perez, O.J.; Hwang, S.; Román, F. Antimicrobial nanomaterials as water disinfectant: Applications, limitations and future perspectives. Sci. Total Environ. 2014, 466–467, 1047–1059.
- Li, J.; Cha, R.; Mou, K.; Zhao, X.; Long, K.; Luo, H.; Zhou, F.; Jiang, X. Nanocellulose-Based Antibacterial Materials. Adv. Healthc. Mater. 2018, 7, e1800334.
