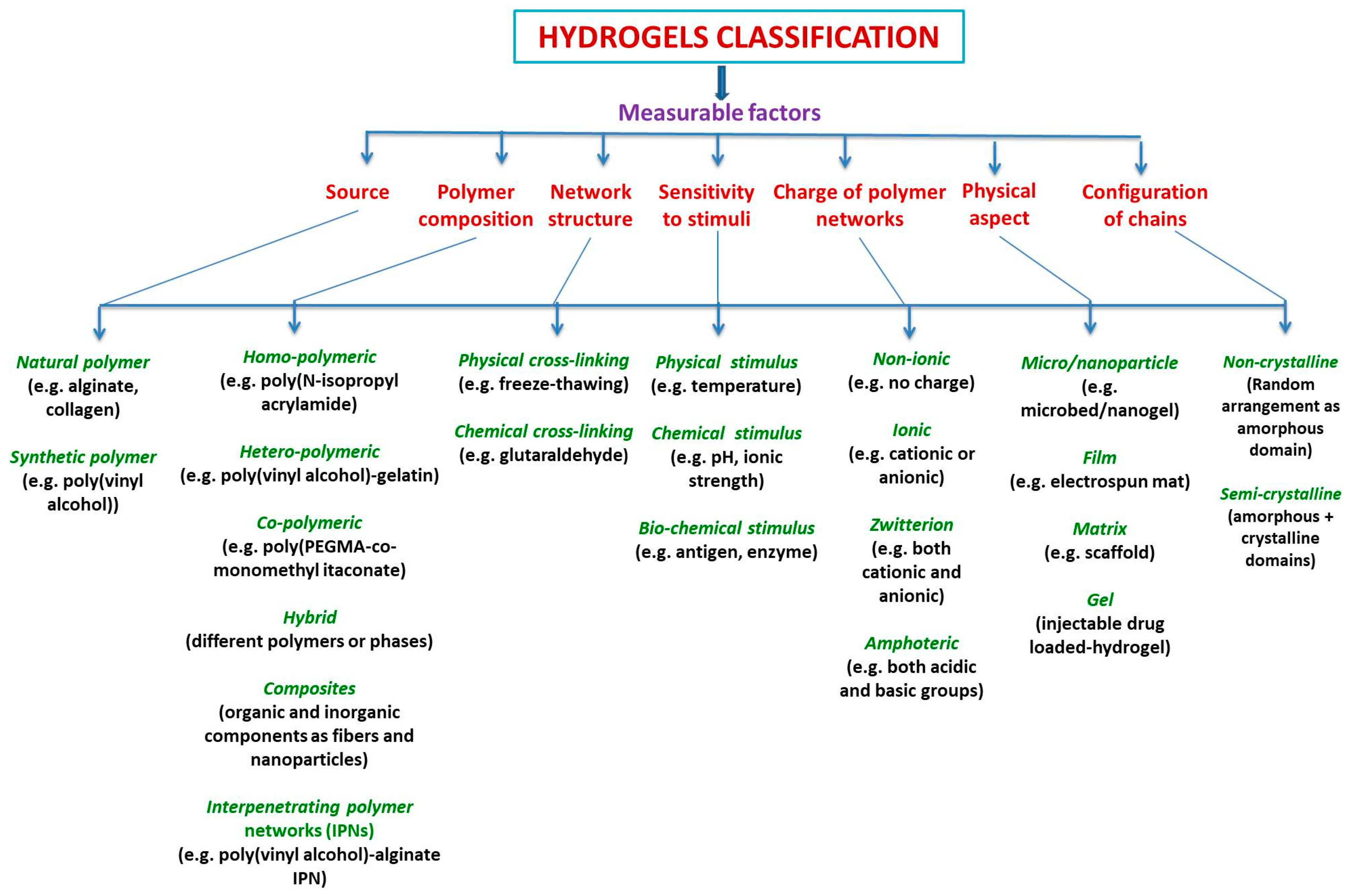
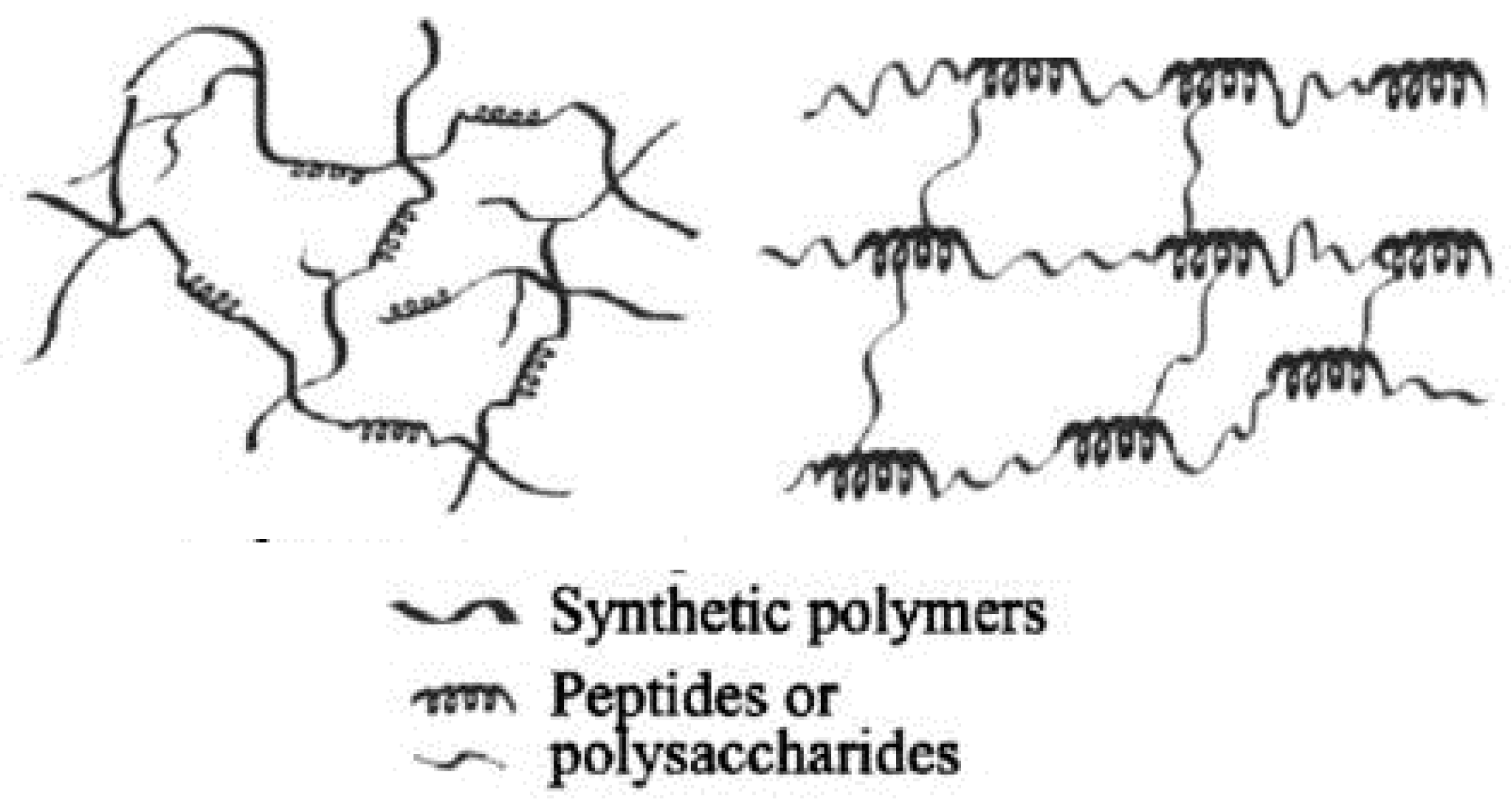
Hybrid hydrogels definition is still debatable. They are defined either as a complex composed chemically or physically cross-linking structures, or it refers to systems combining different polymers and/or with nanoparticles, such as plasmonic, magnetic, and carbonaceous nanoparticles, among others, or they are constituted by chemically, functionally, and morphologically distinct features from at least two different classes of molecules, which can include biologically active polymers as polysaccharides and/or proteins, peptides, or nano/microstructures, interconnected via physical or chemical means.
- organic hybrid polymeric hydrogels
- natural polymers
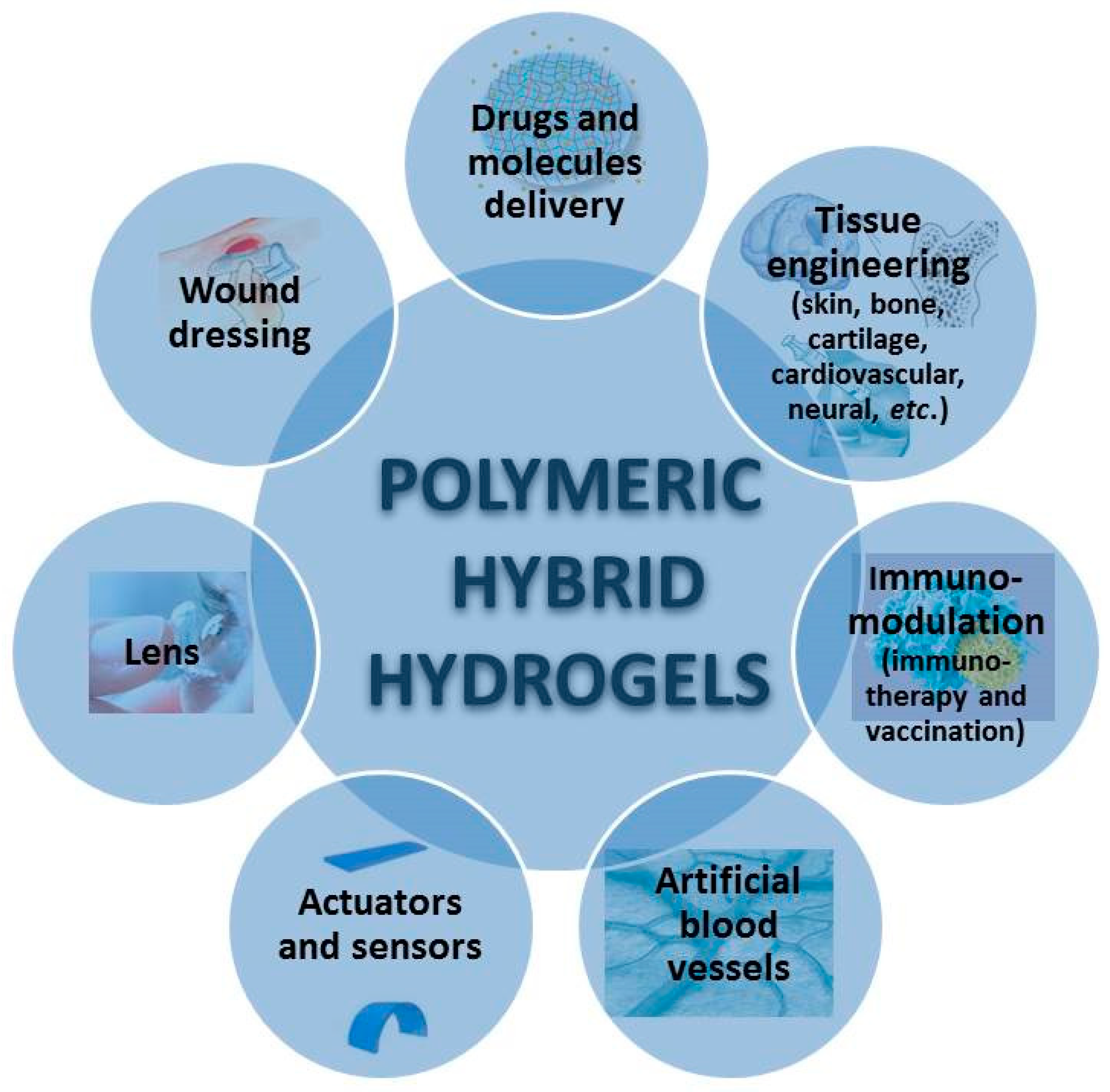
- medical applications
- homopolysaccharides
- heteropolysaccharides
- polypeptides
- proteins
- tissue engineering
- Bone Tissue Engineering
- cartilage Tissue Engineering
- Wound Dressing
- Drug Delivery
- . Immunotherapy
Table of Contents
- Introduction
1.1.Polymers Used in Hybrid Hydrogels
1.2.Types of Hybrid Hydrogels
1.2.1. Microgel
1.2.2. Hybrid Nanogels
1.2.3. Multifunctional Hybrid Nanogels
1.2.4. Hybrid Polymer Nanogel/Hydrogels
1.2.5. Physical Hydrogels
1.2.6. Chemically or Covalently Crosslinked Hydrogels
1.2.7. Self-Assembling Hybrid Hydrogels
1.2.8. Interpenetrated and Semi-Interpenetrated Polymer Networks
1.2.9. Core-Shell Polymer Networks
1.2.10. Supramolecular Hydrogel
2. Preparation Procedures for Polymeric Hybrid Hydrogels
2.1. Routes to Obtain Hybrid Hydrogels
2.1.1.Chemical Modifications
2.1.2. Functionalization
2.1.3. Stealth Functionalization
2.1.4. PEGylation
2.2. Processing Methods
3. Properties
3.3.Swelling
3.2. Mechanical Properties
3.3. Responsiveness
3.4. Porosity and Permeation
- Applications
- Homopolysaccharides-Based Hybrid Hydrogels
5.1. Ability of Homopolysaccharides to Form Hybrid Hydrogels
5.2. Biomedical Applications of Homopolysaccharides-Based Hydrogels
5.2.1. Tissue Engineering
5.2.2. Wound Dressing
5.2.3. Drug Delivery
5.2.4. Other Biomedical Applications of Polysaccharides
- Heteropolysaccharides-Based Hybrid Hydrogels
6.1. Ability of Heteropolysaccharides to Form Hybrid Hydrogels
6.2. Biomedical Applications of Heteropolysaccharides-Based Hybrid Hydrogels
6.2.1. Tissue Engineering
6.2.2. Wound Dressing
6.2.3. Drug Delivery
7. Hybrid Proteins Based Hydrogels for Biomedical Applications
7.1. Ability of Proteins/Peptides to Form Hybrid Hydrogels
7.2. Properties of Proteins to Form Hybrid Hydrogels for Biomedical Applications
7.2.1. Collagen
7.2.2. Gelatin
7.2.3. Keratin
7.2.4. Bovine Serum Albumin
7.2.5. Silk
7.2.6. Resilin
7.2.7. Whey Proteins
7.2.8. Soy Protein Isolate
7.2.9. Elastin
7.3. Biomedical Applications of Protein Based Hybrid Hydrogels
7.3.1. Tissue Engineering
7.3.2. Bone Tissue Engineering
7.3.3. Cartilage Tissue Engineering
7.3.4. Wound Healing
7.3.5. Drug and Molecule Delivery
8. Nucleic Acids Based Hybrid Hydrogels for Biomedical Applications
8.1. Nucleic Acids Ability to Form Hydrogels
8.2. Biomedical Applications of Nucleic Acids-Containing Hybrid Hydrogels
8.2.1. Drug Delivery
8.2.2. Immunotherapy
8.2.3. Biosensing Applications
- Hybrid Hydrogels Containing Lignin for Biomedical Applications
9.1. Bioactive Compounds Delivery
9.2. Applications as Antimicrobial, Antioxidant, Antifungal materials
- Conclusions and Future Trends
1. Introduction
1.1. Polymers Used in Hybrid Hydrogels
Polysaccharides | Polypeptides and Proteins | Polynucleotides and Others | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Homopolysaccharides | Heteropolysaccharides | ||||||||||||
Cellulose and derivatives (carboxymethylcellulose, hydroxyethyl cellulose; hydroxypropylcellulose methylcellulose hydroxypropylmethylcellulose; cellulose acetophphalate) |
|
|
|
| |||||||||
1.1.1. Microgel
1.1.2. Hybrid Nanogels
1.1.3. Multifunctional Hybrid Nanogels
1.1.4. Hybrid Polymer Nanogel/Hydrogels
1.1.5. Physical Hydrogels
1.1.6. Chemically or Covalently Crosslinked Hydrogels
1.1.7. Self-Assembling Hybrid Hydrogels
1.1.8. Interpenetrated and Semi-Interpenetrated Polymer Networks
1.1.9. Core-Shell Polymer Networks
1.1.10. Supramolecular Hydrogel
2. Preparation Procedures for Polymeric Hybrid Hydrogels
2.1. Routes to Obtain Hybrid Hydrogels
2.1.1. Chemical Modifications
2.1.2. Functionalization
2.1.3. Stealth Functionalization
2.1.4. PEGylation
2.2. Processing Methods
3. Properties
3.1. Swelling
3.2. Mechanical Properties
3.3. Responsiveness
3.4. Porosity and Permeation
4. Applications
References
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182.
- Budama-Kilinc, Y.; Cakir-Koc, R.; Aslan, B.; Özkan, B.; Mutlu, H.; Üstün, E. Chapter 12: Hydrogels in Regenerative Medicine. In Biomaterials in Regenerative Medicine; Dobrzański, L.A., Ed.; IntechOpen: London, UK, 2018; pp. 277–301.
- Durmaz, S.; Okay, O. Acrylamide/2-acrylamido-2-methylpropane sulfonic acid sodium salt-based hydrogels: Synthesis and characterization. Polymer 2000, 41, 3693–3704.
- Ekici, S.; Saraydin, D. Synthesis, characterization and evaluation of IPN hydrogels for antibiotic release. Drug Deliv. 2004, 11, 381–388.
- Peppas, N.P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46.
- Swami, S.N. Radiation synthesis of polymeric hydrogels for swelling-controlled drug release studies. Ph.D. Thesis, Western Sydney University, Sydney, Australia, 2004. Available online: http://handle.uws.edu.au:8081/1959.7/698 (accessed on 7 January 2020).
- Katime, I.; Novoa, R.; Zuluaga, F. Swelling kinetics and release studies of theophylline and aminophylline from acrylic acid/n-alkyl methacrylate hydrogels. Eur. Polym. J. 2001, 37, 1465–1471.
- Zhang, T.; Yang, R.; Yang, S.; Guan, J.; Zhang, D.; Ma, Y.; Liu, H. Research progress of self-assembled nanogel and hybrid hydrogel systems based on pullulan derivatives. Drug Deliv. 2018, 25, 278–292.
- Kopeček, J.; Tang, A.; Wang, C.; Stewart, R.J. De novo design of biomedical polymers. Hybrids from synthetic macromolecules and genetically engineered protein domains. Macomol. Symp. 2001, 174, 31–42.
- Molina, M.; Asadian-Birjand, M.; Balach, J.; Bergueiro, J.; Miceliac, E.; Calderon, M. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 2015, 44, 6161–6186.
- Jia, X.; Kiick, K.L. Hybrid multicomponent hydrogels for tissue engineering. Macromol. Biosci. 2009, 9, 140–156.
- Myung, D.; Waters, D.; Wiseman, M.; Duhamel, P.E.; Noolandi, J.; Ta, C.N.; Frank, C.W. Progress in the development of interpenetrating polymer network hydrogels. Polym. Adv. Technol. 2008, 19, 647–657.
- Jonker, A.M.; Löwik, D.W.P.M.; van Hest, J.C.M. Peptide- and protein-based hydrogels. Chem. Mater. 2012, 24, 759–773.
- Lau, H.K.; Kiick, K.L. Opportunities for multicomponent hybrid hydrogels in biomedical applications. Biomacromolecules 2015, 16, 28–42.
- Maisani, M.; Pezzoli, D.; Chassande, O.; Mantovani, D. Cellularizing hydrogel-based scaffolds to repair bone tissue: How to create a physiologically relevant micro-environment? J. Tissue Eng. 2017, 8.
- Joseph, C.A.; McCarthy, C.W.; Tyo, A.; Hubbard, K.; Fisher, H.; Altscheffel, J.; He, W.; Pinnaratip, R.; Lui, Y.; Lee, B.P.; et al. Development of an injectable nitric oxide releasing poly(ethylene) glycol-Fibrin adhesive hydrogel. ACS Biomater. Sci. Eng. 2019, 5, 959–969.
- Hoffman, A.S. Hydrogels for biomedical applications. Ann. N. Y. Acad. Sci. 2001, 944, 62–73.
- Yang, G.; Lin, H.; Rothrauff, B.B.; Yu, S.; Tuan, R.S. Multilayered polycaprolactone/gelatin fiber-hydrogel composite for tendon tissue engineering. Acta Biomater. 2016, 35, 68–76.
- Patel, D.; Sharma, S.; Screen, H.R.C.; Bryant, S.J. Effects of cell adhesion motif, fiber stiffness, and cyclic strainon tenocyte gene expression in a tendon mimetic fiber composite hydrogel. Biochem. Biophys. Res. Commun. 2018, 499, 642–647.
- Kopeček, J. Hydrogel Biomaterials: A Smart Future? Biomaterials 2007, 28, 5185–5192.
- Liang, Y.; Liu, W.; Han, B.; Yang, C.; Ma, Q.; Song, F.; Bi, Q. An in situ formed biodegradable hydrogel for reconstruction of the corneal endothelium. Colloids Surf. B: Biointerfaces 2011, 82, 1–7.
- Ehrick, J.D.; Deo, S.K.; Browning, T.W.; Bachas, L.G.; Madou, M.J.; Daunert, S. Genetically engineered protein in hydrogels tailors stimuli-responsive characteristics. Nat. Mater. 2005, 4, 298–302.
- Sengupta, D.; Heilshorn, S.C. Protein-engineered biomaterials: Highly tunable tissue engineering scaffolds. Tissue Eng. Part B: Rev. 2010, 16, 285–293.
- Foo, W.P.C.T.; Lee, J.S.; Mulyasasmita, W.; Parisi-Amon, A.; Heilshorn, S.C. Two-component protein-engineered physical hydrogels for cell encapsulation. Proc. Natl. Acad. Sci. USA 2009, 106, 22067–22072.
- Sakai, S.; Hirose, K.; Taguchi, K.; Ogushi, Y.; Kawakami, K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials 2009, 30, 3371–3377.
- Xing, Y.; Cheng, E.; Yang, Y.; Chen, P.; Zhang, T.; Sun, Y.; Yang, Z.; Liu, D. Self-assembled DNA hydrogels with designable thermal and enzymatic responsiveness. Adv. Mater. 2011, 23, 1117–1121.
- Davidenko, N.; Campbell, J.J.; Thian, E.S.; Watson, C.J.; Cameron, R.E. Collagen–hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010, 6, 3957–3968.
- Raschip, I.E.; Hitruc, G.E.; Vasile, C.; Popescu, M.C. Effect of the lignin type on the morphology and thermal properties of xanthan/lignin hydrogels. Int. J. Biol. Macromol. 2013, 54, 230–237.
- Raschip, I.E.; Hitruc, E.G.; Vasile, C. Semi-interpenetrating polymer networks containing polysaccharides. II. Xanthan/lignin network: A spectral and thermal characterization. High Perform. Polym. 2011, 23, 219–229.
- Hejčl, A.; Sedý, J.; Kapcalová, M.; Toro, D.A.; Amemori, T.; Lesný, P.; Likavcanová-Mašínová, K.; Krumbholcová, E.; Prádný, M.; Michálek, J.; et al. HPMA-RGD hydrogels seeded with mesenchymal stem cells improve functional outcome in chronic spinal cord injury. Stem Cells Dev. 2010, 19, 1535–1546.
- Beamish, J.A.; Zhu, J.; Kottke-Marchant, K.; Marchant, R.E. The effects of monoacrylated poly(ethylene glycol) on the properties of poly (ethylene glycol) diacrylate hydrogels used for tissue engineering. J. Biomed. Mater. Res. Part A 2010, 92, 441–450.
- Zustiak, S.P.; Leach, J.B. Hydrolytically degradable poly (ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules 2010, 11, 1348–1357.
- Silva, A.K.A.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.-W. Growth factor delivery approaches in hydrogels. Biomacromolecules 2008, 10, 9–18.
- Varghese, S.; Elisseeff, J.H. Hydrogels for musculoskeletal tissue engineering. Adv. Polym. Sci. 2006, 203, 95–144.
- Jiang, Z.; Hao, J.; You, Y.; Liu, Y.; Wang, Z.; Deng, X. Biodegradable and thermoreversible hydrogels of poly (ethylene glycol)-poly (ε-caprolactone-co-glycolide)-poly (ethylene glycol) aqueous solutions. J. Biomed. Mater. Res. Part A 2008, 87, 45–51.
- Deshmukh, M.; Singh, Y.; Gunaseelan, S.; Gao, D.; Stein, S.; Sinko, P.J. Biodegradable poly (ethylene glycol) hydrogels based on a self-elimination degradation mechanism. Biomaterials 2010, 31, 6675–6684.
- Schmedlen, R.H.; Masters, K.S.; West, J.L. Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials 2002, 23, 4325–4332.
- Ossipov, D.A.; Brännvall, K.; Forsberg-Nilsson, K.; Hilborn, J. Formation of the first injectable poly (vinyl alcohol) hydrogel by mixing of functional PVA precursors. J. Appl. Polym. Sci. 2007, 106, 60–70.
- Higuchi, A.; Aoki, N.; Yamamoto, T.; Miyazaki, T.; Fukushima, H.; Tak, T.M.; Jyujyoji, S.; Egashira, S.; Matsuoka, Y.; Natori, S.H. Temperature-induced cell detachment on immobilized pluronic surface. J. Biomed. Mater. Res. Part A 2006, 79, 380–392.
- Zhu, J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656.
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484.
- Matsumoto, T.; Isogawa, Y.; Tanaka, T.; Kondo, A. Streptavidin-hydrogel prepared by sortase A-assisted click chemistry for enzyme immobilization on an electrode. Biosens. Bioelectron. 2018, 99, 56.
- Ito, F.; Usui, K.; Kawahara, D.; Suenaga, A.; Maki, T.; Kidoaki, S.; Suzuki, H.; Taiji, M.; Itoh, M.; Hayashizaki, Y.; et al. Reversible hydrogel formation driven by protein-peptide-specific interaction and chondrocyte entrapment. Biomaterials 2009, 31, 58–66.
- Ramirez, M.; Guan, D.; Ugaz, V.; Chen, Z. Intein-triggered artificial protein hydrogels that support the immobilization of bioactive proteins. J. Am. Chem. Soc. 2013, 135, 5290.
- Krishna, O.D.; Kiick, K.L. Protein- and peptide-modified synthetic polymeric biomaterials. Biopolymers 2010, 94, 32–48.
- Pamfil, D.; Vasile, C.; Tarțău, L.; Vereștiuc, L.; Poieată, A. pH-Responsive 2-hydroxyethyl methacrylate/citraconic anhydride-modified collagen hydrogels as ciprofloxacin carriers for wound dressings. J. Bioact. Compat. Polym. 2017, 32, 355–381.
- Dorwal, D. Nanogels as Novel and versatile pharmaceutics. J. Pharm. Pharm. Sci. 2012, 4, 67–74.
- Yadav, H.K.S.; Al Halabi, N.A.; Alsalloum, G.A. Nanogels as Novel Drug Delivery Systems-A Review. J. Pharm. Pharm. Res. 2017, 1, 1–8.
- Tahara, Y.; Akiyoshi, K. Current advances in self-assembled nanogel delivery systems for immunotherapy. Adv. Drug Deliv. Rev. 2015, 95, 65–76.
- Bencherif, S.A.; Siegwart, D.J.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Washburn, N.R.; Matyjaszewski, K. Nanostructured hybrid hydrogels prepared by a combination of atom transfer radical polymerization and free radical polymerization. Biomaterials 2009, 30, 5270–5278.
- Sahiner, N.; Godbey, W.T.; McPherson, G.L.; John, V.T. Microgel, nanogel and hydrogel–hydrogel semi-IPN composites for biomedical applications: Synthesis and characterization. Colloid Polym. Sci. 2006, 284, 1121–1129.
- Wu, W.; Zhou, S. Hybrid micro-/nanogels for optical sensing and intracellular imaging. Nano Rev. 2010, 1, 5730.
- Lohani, A.; Singh, G.; Sankar Bhattacharya, S.; Verma, A. Interpenetrating Polymer Networks as Innovative Drug Delivery Systems. J. Drug Deliv. 2014, 2014, 583612.
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007.
- Ebara, M.; Kotsuchibashi, Y.; Narain, R.; Idota, N.; Kim, Y.J.; Hoffman, J.M.; Uto, K.; Aoyagit, T. Smart Biomaterials; Springer: Tokyo, Japan, 2014; ISBN 978-4-431-54400-5.
- Amini, A.A.; Nair, L.S. Injectable hydrogels for bone and cartilage repair. Biomed. Mater. 2012, 7, 024105.
- Jin, R. Chapter 2: In-Situ Forming Biomimetic Hydrogels for Tissue Regeneration. In Biomedicine; Lin, C., Ed.; INTECH Open Access Publisher: London, UK, 2012; pp. 35–58.
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633.
- Czarnecki, S.; Rossow, T.; Seiffert, S. Hybrid Polymer-Network Hydrogels with Tunable Mechanical Response. Polymers 2016, 8, 82.
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136.
- Lin, P.; Ma, S.; Wang, X.; Zhou, F. Molecularly engineered dual-crosslinked hydrogel with ultrahigh mechanical strength, toughness, and good self-recovery. Adv. Mater. 2015, 27, 2054–2059.
- Narita, T.; Mayumi, K.; Ducouret, G.; Hébraud, P. Viscoelastic properties of poly(vinyl alcohol) hydrogels having permanent and transient cross-links studied by microrheology, classical rheometry, and dynamic light scattering. Macromolecules 2013, 46, 4174–4183.
- Kondo, S.; Hiroi, T.; Han, Y.S.; Kim, T.H.; Shibayama, M.; Chung, U.I.; Sakai, T. Reliable hydrogel with mechanical “fuse link” in an aqueous environment. Adv. Mater. 2015, 27, 7407–7411.
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. 2009, 48, 6974–6998.
- Kopeček, J.; Yang, J. Smart Self-Assembled Hybrid Hydrogel Biomaterials. Angew. Chem. Int. Ed. 2012, 51, 7396–7417.
- Rodriguez, L.M.D.L.; Hemar, Y.; Cornish, J.; Brimble, M.A. Structure–mechanical property correlations of hydrogel forming β-sheet peptides. Chem. Soc. Rev. 2016, 45, 4797.
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension–how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024.
- Yang, J.; Xu, C.; Wang, C.; Kopeček, J. Refolding hydrogels self-assembled from N-(2-hydroxypropyl)methacrylamide graft copolymers by antiparallel coiled-coil formation. Biomacromolecules 2006, 7, 1187–1195.
- Ren, N.; Sun, R.; Xia, K.; Zhang, Q.; Li, W.; Wang, F.; Zhang, X.; Ge, Z.; Wang, L.; Fan, C.; et al. DNA-Based Hybrid Hydrogels Sustain Water-Insoluble Ophthalmic Therapeutic Delivery against Allergic Conjunctivitis. ACS Appl. Mater. Interfaces 2019, 11, 26704–26710.
- Mundargi, R.C.; Patil, S.A.; Kulkarni, P.V.; Mallikarjuna, N.N.; Aminabhavi, T.M. Sequential interpenetrating polymer network hydrogel microspheres of poly(methacrylic acid) and poly(vinyl alcohol) for oral controlled drug delivery to intestine. J. Microencapsul. 2008, 25, 228–240.
- Liu, X.; Guo, H.; Zha, L. Study of pH/temperature dual stimuli-responsive nanogels with interpenetrating polymer network structure. Polym. Int. 2012, 61, 1144–1150.
- Blackburn, W.H.; Dickerson, E.B.; Smith, M.H.; McDonald, J.F.; Lyon, L.A. Peptide-Functionalized Nanogels for Targeted siRNA Delivery. Bioconjug. Chem. 2009, 20, 960–968.
- Schachschal, S.; Balaceanu, A.; Melian, C.; Demco, D.E.; Eckert, T.; Richtering, W.; Pich, A. Polyampholyte Microgels with Anionic Core and Cationic Shell. Macromolecules 2010, 43, 4331–4339.
- Zhang, W.; Yao, R.; Tao, W.; He, H.; Shui, S. Preparation of monodisperse HPMC/PAA hybrid nanogels via surfactant-free seed polymerization. Colloid Polym. Sci. 2013, 292, 317–324.
- Nayak, S.; Lee, H.; Chmielewski, J.; Lyon, L.A. Folate-Mediated Cell Targeting and Cytotoxicity Using Thermoresponsive Microgels. J. Am. Chem. Soc. 2004, 126, 10258–10259.
- Dickerson, E.; Blackburn, W.; Smith, M.; Kapa, L.; Lyon, L.A.; McDonald, J. Chemosensitization of cancer cells by siRNA using targeted nanogel delivery. BMC Cancer 2010, 10, 10.
- Lapeyre, V.; Ancla, C.; Catargi, B.; Ravaine, V. Glucose-responsive microgels with a core-shell structure. J. Colloid Interface Sci. 2008, 327, 316–323.
- Zieris, A.; Prokoph, S.; Levental, K.R.; Welzel, P.B.; Grimmer, M.; Freudenberg, Y.; Werner, C. FGF-2 and VEGF functionalization of starPEG–heparin hydrogels to modulate biomolecular and physical cues of angiogenesis. Biomaterials 2010, 31, 7985–7994.
- Jin, R.; Moreira Teixeira, L.S.; Krouwels, A.; Dijkstra, P.J.; van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Synthesis and characterization of hyaluronic acid–poly (ethylene glycol) hydrogels via Michael addition: An injectable biomaterial for cartilage repair. Acta Biomater. 2010, 6, 1968–1977.
- Li, F.; Griffith, M.; Li, Z.; Tanodekaew, S.; Sheardown, H.; Hakim, M.; Carlsson, D.J. Recruitment of multiple cell lines by collagen-synthetic copolymer matrices in corneal regeneration. Biomaterials 2005, 26, 3093–3104.
- Nistor, M.T.; Vasile, C.; Tatia, R.; Chiriac, A. Hybrid collagen/pNIPAAM hydrogel nanocomposites for tissue engineering application. Colloid Polym. Sci. 2018, 296, 1555–1571.
- Nistor, M.T.; Chiriac, A.P.; Nita, L.E.; Vasile, C. Characterization of the semi-interpenetrated network based on collagen and poly(N-isopropyl acrylamide-co-diethylene glycol diacrylate). Int. J. Pharm. 2013, 452, 92–101.
- Cheaburu, C.N.; Vasile, C. Responsive freeze-drying interpolymeric associations of alginic acid and PNIPAM. II. Transition and temperature dependence on pH and composition. Cell. Chem. Technol. 2008, 42, 207–212.
- Radvar, E.; Azevedo, H.S. Supramolecular Peptide/Polymer Hybrid Hydrogels for Biomedical Applications. Macromol. Biosci. 2019, 19, 1800221.
- Vandermeulen, G.W.M.; Klok, H.A. Peptide/protein hybrid materials: Enhanced control of structure and impropved performance through conjugation of biological and synthetic polymers. Macromol. Biosci. 2004, 4, 383–398.
- Gulrez, S.K.H.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation, Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering. Analysis and Modeling to Technology Applications; Carpi, A., Ed.; IntechOpen: Rijeka, Croatia, 2011.
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092.
- Barner-Kowollik, C. Handbook of RAFT Polymerization; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2008; ISBN 978-3-527-31924-4.
- Wang, C.; Kopeček, J.; Stewart, R.J. Hybrid hydrogels crosslinked by genetically engineered coiled-coil block proteins. Biomacromolecules 2001, 2, 912–920.
- Lim, S.; Jung, G.A.; Muckom, R.J.; Glover, D.J.; Clark, D.S. Engineering bioorthogonal protein–polymer hybrid hydrogel as a functional protein immobilization platform. Chem. Commun. 2019, 55, 806–809.
- Sierra-Martin, B.; Fernandez-Barbero, A. Multifunctional hybrid nanogels for theranostic applications. Soft Matter 2015, 14, 8205–8216.
- Sanson, N.; Rieger, J. Synthesis of nanogels/microgels by conventional and controlled radical crosslinking copolymerization. Polym. Chem. 2010, 1, 96577.
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429.
- Wu, H.Q.; Wang, C.C. Biodegradable smart nanogels: A new platform for targeting drug delivery and biomedical diagnostics. Langmuir 2016, 32, 6211–6225.
- Karg, M. Functional Materials Design through Hydrogel Encapsulation of Inorganic Nanoparticles: Recent Developments and Challenges. Macromol. Chem. Phys. 2016, 217, 242–255.
- Eslami, P.; Rossi, F.; Fedeli, S. Review Hybrid Nanogels: Stealth and Biocompatible Structures for Drug Delivery Applications. Pharmaceutics 2019, 11, 71.
- Grover, G.N.; Rao, N.; Christman, K.L. Myocardial Matrix-Polyethylene Glycol Hybrid Hydrogels for Tissue Engineering. Nanotechnology 2014, 25, 014011.
- Foster, J. Chapter 12: PEGylation and BioPEGylation of Polyhydroxyalkanoates: Synthesis, Characterisation and Applications. In Biopolymers; Elnashar, M., Ed.; IntevhOpen: Rijeka, Croatia, 2010; pp. 243–256.
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials 2014, 35, 4969–4985.
- Chirani, N.; Yahia, L.H.; Gritsch, L.; Motta, F.L.; Chirani, S.; Faré, S. History and Applications of Hydrogels. J. Biomed. Sci. 2015, 4, 32.
- Zhou, M.; Smith, A.M.; Das, A.K.; Hodson, N.W.; Collins, R.F.; Ulijn, R.V.; Gough, J.E. Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells. Biomaterials 2009, 30, 2523–2530.
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157.
- Bakarich, S.E.; Balding, P.; Gorkin, R.; Spinks, G.M. Printed ioniccovalent entanglement hydrogels from carrageenan and an epoxy amine. RSC Adv. 2014, 4, 38088–38092.
- Tan, H.; Marra, K.G. Injectable, Biodegradable Hydrogels for Tissue Engineering Applications. Materials 2010, 3, 1746–1767.
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360.
- Weber, L.M.; Lopez, C.G.; Anseth, K.S. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. J. Biomed. Mater. Res. A 2009, 90, 720–729.
- Fujioka-Kobayashi, M.; Ota, M.S.; Shimoda, A.; Nakahama, K.; Akiyoshi, K.; Miyamoto, Y.; Iseki, S. Cholesteryl group- and acryloyl group-bearing pullulan nanogel to deliver BMP2 and FGF18 for bone tissue engineering. Biomaterials 2012, 33, 7613–7620.
- Shimoda, A.; Chen, Y.; Akiyoshi, K. Nanogel containing electrospun nanofibers as a platform for stable loading of proteins. RSC Adv. 2016, 6, 40811–40817.
- Shimoda, A.; Sawada, S.-i.; Akiyoshi, K. Intracellular protein delivery using self-assembled amphiphilic polysaccharide nanogels. In Intracellular Delivery II: Fundamentals and Applications; Prokop, A., Iwasaki, Y., Harada, A., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 265–274.
- Molinos, M.; Carvalho, V.; Silva, D.M.; Gama, F.M. Development of a Hybrid Dextrin Hydrogel Encapsulating Dextrin Nanogel As Protein Delivery Systems. Biomacromolecules 2012, 13, 517–527.
- Lee, S.C.; Kwon, I.K.; Park, K. Hydrogels for delivery of bioactive agents: A historical perspective. Adv. Drug Deliv. Rev. 2013, 65, 17–20.
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273.
- Yom-Tov, O.; Neufeld, L.; Seliktar, D.; Bianco-Peled, H. A novel design of injectable porous hydrogels with in situ pore formation. Acta Biomater. 2014, 10, 4236–4246.
- Abebe, D.G.; Fujiwara, T. Controlled thermoresponsive hydrogels by stereocomplexed PLA-PEG-PLAprepared via hybrid micelles of premixed copolymers with different PEG lengths. Biomacromolecules 2012, 13, 1828–1836.
- Sefton, M.V.; May, M.H.; Lahooti, S.; Babensee, J.E. Making microencapsulation work: Conformal coating, immobilization gels and in vivo performance. J. Control. Release 2000, 65, 173–186.
- Wang, L.; Guo, S.; Dong, J.; Cui, J.; Hao, J. Microgels in biomaterials and nanomedicines. Adv. Colloid Interface Sci. 2019, 266, 1–20.