Metal/carbon nanotube (CNT) composites are promising functional materials due to the various superior properties of CNTs in addition to the characteristics of metals. Electrochemical deposition can be classified into three types: (1) composite plating by electrodeposition or electroless deposition, (2) metal coating on CNT by electroless deposition, and (3) electrodeposition using CNT templates, such as CNT sheets and CNT yarns.
- metal/carbon nanotube composite
- electrochemical deposition
- electrodeposition
- electroless deposition
- composite plating
- carbon nanotube sheet
- carbon nanotube yarn
1. Introduction
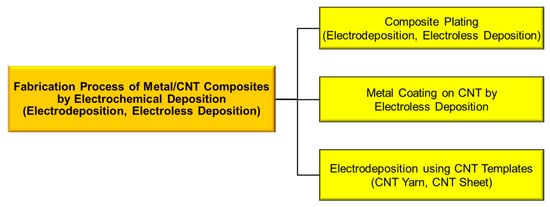
2. Fabrication of Metal/CNT Composites Using Composite Plating by Electrodeposition or Electro Less Deposition
2.1. Composite Plating
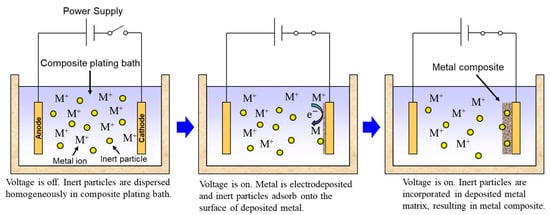
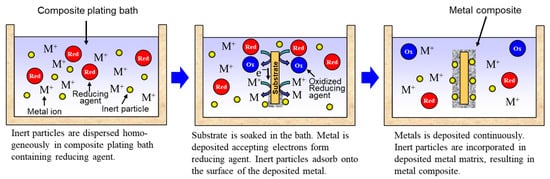
Rough schematics of composite plating by electrodeposition and electroless deposition are displayed in Figure 32 and Figure 43, respectively. In the case of electrodeposition, inert particles are dispersed homogeneously in a plating bath. When a voltage is applied, metal is electrodeposited on a cathode and the particles adsorb on the surface of the deposited metal. Then, the particles are embedded in depositing metal, resulting in a metal composite (Figure 32). In the case of CNT composite plating by electrodeposition, inert particles are dispersed homogeneously in a plating bath containing a reducing agent. When a substrate is soaked in the bath, metal is reductively deposited on the substrate accepting electrons from the reducing agent and, at the same time, the particles adsorb on the surface of the deposited metal. The particles are then embedded in depositing metal, resulting in a metal composite (Figure 43). In general, the substrate is pre-treated and catalyst particles, such as Pd particles, are fixed on the surface of the substrate before soaking into the plating bath. As far as was searched, the first article of the composite plating is on Cu/graphite composites by electrodeposition and was reported in 1928 [4][3]. Regarding the mechanism of the composite plating, several models have been proposed [5,6,7,8,9][4][5][6][7][8].
2.2. Preparation of Plating Bath for Metal/CNT Composite Plating
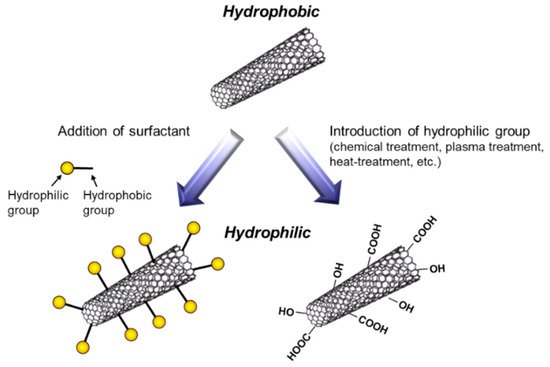
To fabricate metal/CNT composites with uniform distribution of CNTs, the preparation of plating baths with homogeneous dispersion of CNTs is important. In general, plating baths are aqueous solutions, while CNTs are hydrophobic. Therefore, hydrophilization of CNTs have been examined by the addition of surfactants or the direct introduction of hydrophilic groups on the surfaces of CNTs (Figure 54). The addition of surfactants in plating baths is a common method. Various kinds of surfactants [11[9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26],12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28], such as sodium dodecylbenzene sulfonate and sodium deoxycholate, have been examined for the homogeneous dispersion of CNTs in a pure water. However, effective surfactants for the dispersion in a pure water are not always effective in plating baths which contain great amounts of ions. Moreover, even if the surfactant is effective for the dispersion of CNTs in a plating bath, CNTs are not always co-deposited by electrochemical deposition. Therefore, the selection of appropriate surfactants is essential. Since the surfactants are likely incorporated in deposited metal matrix during electrochemical deposition, the concentration of surfactants should be examined. On the contrary, the direct introduction of hydrophilic groups, such as -COOH, onto the surfaces of CNTs has been examined using a chemical treatment [29][27], a plasma treatment [30][28], a heat treatment [31][29], and so on. These methods destroy the sp2 carbon bonding of the surfaces of CNTs. Therefore, the conditions of the treatments should be examined.
2.3. Unique Feature of Composite Plating Using CNTs as Inert Particles
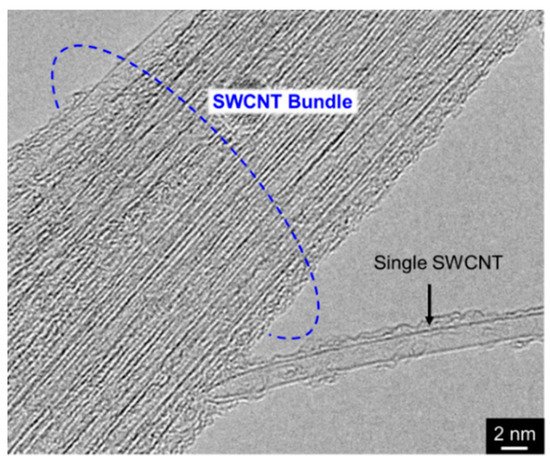
2.3. Unique Feature of Composite Plating Using CNTs as Inert Particles
Since a single CNT, especially multi-walled CNT (MWCNT) has a fibrous shape with large aspect ratio in addition to a high electrical conductivity in the axis direction. Therefore, composite plating using CNTs as inert particles often shows a unique feature unlike other composite plating using insulation particles such as Al2O3 particles. The schematic of the unique feature is showed in Figure 76 [34][30]. When a part of a MWCNT is incorporated in the deposited metal matrix during electrodeposition, the metal can be electrodeposited not only on the deposited metal but also on the protruding edge (a defect site) of the MWCNT. If the defect sites exist on the sidewall of the MWCNT, the metal can also be electrodeposited on the defect sites.
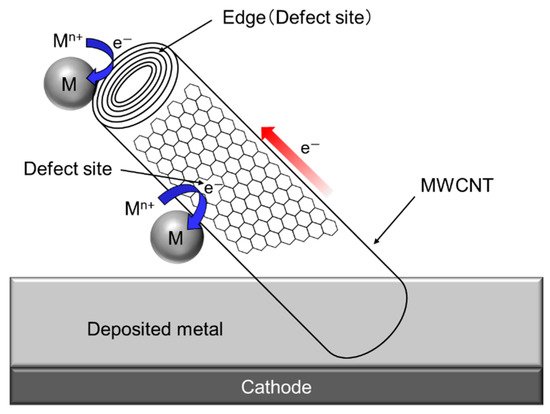
2.4. Fabrication of Metal/CNT Composites Using Composite Plating by Electrodeposition
Fabrication conditions in these articles are listed in Table 1.
2.5. Fabrication of Metal/CNT Composites Using Composite Plating by Electroless Deposition
| Metal | CNT | Treatment of CNT | Base Plating Bath | Surfactant | Remarks | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Ni | MWCNT | Chemical treatment | Dull Watts bath | Sodium lauryl sulfate | Corrosion behavior | 2020 | [32] |
| Ni | MWCNT | Chemical treatment | Dull Watts bath | Sodium lauryl sulfate | Corrosion protection | 2020 | [33] |
| Ni | MWCNT | Wrapped by polydopamine | Dull Watts bath | Non | Wear and corrosion resistance | 2019 | [34] |
| Ni | MWCNT | Non | Ionic liquid (choline chloride/carbamide) | Non | Non-aqueous solvent | 2017 | [35] |
| Ni | MWCNT | Non | Sulfamate bath | Cationic surfactant, compound name is unknown | Improvement in tool life | 2014 | [36] |
| Ni | MWCNT | Non | NiSO4+NaCl | Polyvinylpyrrolidone | Cyclic voltametric route | 2011 | [37] |
| Ni | MWCNT | Ball milling | Bright Watts bath | Sodium lauryl sulfate and Hydroxypropylcellulose |
Corrosion behavior | 2011 | [38] |
| Ni | MWCNT | Chemical treatment | Choline chloride/urea | Non | Non-aqueous solvent | 2010 | [39] |
| Ni | MWCNT | Non | Bright Watts bath | Polyacrylic acid | Solid lubrication | 2008 | [40] |
| Ni | MWCNT | Ball milling | Watts type bath | Sodium lauryl sulfate, Cetyltrimethylammonium bromide | Effects of surfactants | 2008 | [41] |
| Ni | MWCNT | Chemical treatment | Dull Watts bath | Non | Effects of current density | 2008 | [42] |
| Ni | MWCNT | Ball milling | Bright Watts bath | Sodium lauryl sulfate and Hydroxypropylcellulose |
Mechanical properties | 2008 | [43] |
| Ni | MWCNT | Non | Bright Watts bath | Non | Mechanical properties | 2008 | [44] |
| Ni | MWCNT | Non | Bright Sulfamate bath | Polyacrylic acid | Low internal stress | 2007 | [45] |
| Ni | MWCNT | Non | Dull Watts bath | Non | Pulse-reverse parameter | 2007 | [46] |
| Ni | MWCNT | Non | Bright Watts bath | Polyacrylic acid | Thermal conductivity | 2006 | [47] |
| Ni | MWCNT | Non | Dull Watts bath | Poly(diallyldimethylammonium chrolide) | Pulse-reverse electrodeposition | 2005 | [48] |
| Ni | MWCNT | Chemical treatment | Dull Watts bath | Cetyltrimethylammonium bromide | Corrosion behavior | 2005 | [49] |
| Ni | MWCNT | Non | Dull Watts bath | Polyacrylic acid | Ni deposition on incorporated CNT | 2004 | [30] |
| Ni | MWCNT | Ball milling | Dull Watts bath | Non | CNT content | 2002 | [50] |
| Ni | MWCNT | Ball milling | Dull Watts bath | Non | Tribological property | 2001 | [51] |
| Ni-Co | MWCNT | Chemical treatment | Dull Watts bath + Co salt |
Non | Corrosion behavior | 2019 | [52] |
| Ni-P | MWCNT | Non | Dull Watts bath + citric acid + P compound | Polyacrylic acid | Tribological properties | 2010 | [53] |
| Ni-Co | MWCNT | Non | Dull Watts bath + Co salt | Compound name is unknown |
Mechanical and tribological properties | 2006 | [54] |
| Ni-P | MWCNT | Non | Ni salts + citric acid + P compounds | Compound name is unknown |
Corrosion properties | 2004 | [55] |
| Cu | MWCNT | Chemical treatment | Citric bath | Non | Corrosion behavior | 2021 | [56] |
| Cu | MWCNT | Chemical treatment | Sulfate bath | Non | Pulse reverse, electrical conductivity | 2020 | [57] |
| Cu | MWCNT | Chemical treatment? | Sulfate bath | Non-ionic surfactants, Compound name is unknown | Mechanical properties, Microlaminated structure | 2020 | [58] |
| Cu | SWCNT | Non | Sulfate bath | Stearyltrimethylammonium chloride | Mechanical properties | 2020 | [59] |
| Cu | SWCNT | Non | Sulfate bath | Non | Microstructure | 2019 | [60] |
| Cu | MWCNT | Non | Sulfate bath | Sodium lauryl sulfate | Jet electrodeposition, Tribological properties |
2019 | [61] |
| Cu | MWCNT | Non | Sulfate bath | Polyacrylic acid | Current collector for LIB anode | 2019 | [62] |
| Cu | MWCNT | Chemical treatment | Sulfate bath | Stearyltrimethylammonium bromide | Electrical conductivity, Corrosion resistance | 2018 | [63] |
| Cu | MWCNT | Non | Sulfate bath | Non-ionic surfactants, Compound name is unknown | Mechanical properties, Laminated structure | 2018 | [64] |
| Cu | MWCNT | Chemical treatment | Sulfate bath | Non | Cu/CNT powder + powder metallurgy | 2018 | [65] |
| Cu | MWCNT | Chemical treatment | Sulfate bath | Non | Cu/CNT powder + powder metallurgy | 2018 | [66] |
| Cu | MWCNT | Chemical treatment | Sulfate bath | Non | Cu/CNT powder + powder metallurgy | 2017 | [67] |
| Cu | MWCNT | Chemical treatment | Commercially available | Nano diamond | Periodic pulse reverse electrodeposition | 2016 | [68] |
| Cu | MWCNT | Non | Sulfate bath | Polyacrylic acid | Current collector for LIB anode | 2016 | [69] |
| Cu | MWCNT | Non | Sulfate bath | Polyacrylic acid | Co-deposition mechanism of CNT | 2013 | [70] |
| Cu | MWCNT | Non | Sulfate bath | Non | Electrochemical reduction behavior | 2011 | [71] |
| Cu | MWCNT | Non | Sulfate bath | Polyacrylic acid | Pulse-reverse | 2011 | [72] |
| Cu | MWCNT | Non | Sulfate bath | Polyacrylic acid | Surface morphology, Hardness, Internal stress | 2010 | [73] |
| Cu | MWCNT | Non | Sulfate bath | Polyacrylic acid | Patterned field emitter | 2008 | [74] |
| Cu | SWCNT | Non | Sulfate bath | Commercial products | Mechanical properties | 2008 | [75] |
| Cu | SWCNT | Chemical treatment | Sulfate bath | Cetyltrimethylammonium chloride | Mechanical properties | 2008 | [76] |
| Cu | Cup-stacked CNT | Non | Sulfate bath | Polyacrylic acid | Various CNTs | 2005 | [77] |
| Cu | MWCNT | Non | Sulfate bath | Polyacrylic acid | Microstructure | 2004 | [78] |
| Cu | MWCNT | Non | Sulfate bath | Polyacrylic acid | Cu/MWCNT composite powder | 2003 | [31] |
| Zn | MWCNT | Chemical treatment | Sulfate bath | Cetyltrimethylammonium bromide | Corrosion resistance | 2021 | [79] |
| Zn | MWCNT | Non | Zincate bath | Unknown | Pulse electrodeposition, Corrosion resistance | 2020 | [80] |
| Zn | MWCNT | Chemical treatment | Sulfate bath | Cetyltrimethylammonium bromide | Corrosion resistance | 2007 | [81] |
| Zn-Ni | MWCNT | Non | Chloride bath | Non | Pulse reverse, Tribological and Corrosion properties | 2016 | [82] |
| Cr | MWCNT | Non | Trivalent Cr bath | Sodium lauryl sulfate | Tribological properties, Corrosion resistance |
2020 | [83] |
| Cr | MWCNT | Non | Trivalent Cr bath | Sodium lauryl sulfate | Tribological properties | 2018 | [84] |
| Cr | MWCNT | Non | Trivalent Cr bath | Non | Mechanical properties | 2009 | [85] |
| Co | MWCNT | Non | Choline chloride/urea | Non | Non-aqueous solvent | 2017 | [86] |
| Co | MWCNT | Non | Sulfate bath | Polyacrylic acid | Field emission properties | 2013 | [87] |
| Co | MWCNT | Non | Sulfate bath | Polyacrylic acid | Tribological properties | 2013 | [88] |
| Co | MWCNT | Acid-treatment | Sulfate bath + citrate | Sodium lauryl sulfate | Tribological properties, Corrosion properties | 2013 | [89] |
| Co-W | MWCNT | Non | Co salt + Tungstate + Citrate | Polyacrylic acid | Tribological properties Corrosion properties |
2015 | [90] |
| Co-W | MWCNT | Non | Co salt + Tungstate + Citrate | Polyacrylic acid | Tribological properties | 2013 | [91] |
| Au | MWCNT | Non | Sulfite bath | Stearyltrimethylammonium chloride | Electrical conductivity, Tribological properties | 2009 | [92] |
| Ag | MWCNT | Non | Choline chloride + glycerol | Poly (N-vinyl pyrrolidone) | Pulse reverse electrodeposition | 2021 | [93] |
| Ag | MWCNT | Non | Iodide bath | Non | Electrical contact resistance against H2S gas | 2021 | [94] |
| Ag | MWCNT | Non | Iodide bath | Non | Hardness, Electrical and Tribological properties | 2020 | [95] |
| Ag | MWCNT | Non | Cyanide bath | Unknown | Electrical contact resistance against H2S gas | 2010 | [96] |
| Al | MWCNT | Acid treatment | Diethylene glycol dimethyl ether | Non | Hardness | 2020 | [97] |
| Al | MWCNT | Non | 1-ethyl-3-methylimidazolium chloride | Non | Hardness | 2006 | [98] |
| Sn | MWCNT | Non | Choline chloride + ethylene glycole | Non | Nucleation study | 2019 | [99] |
| Pb-Sn | MWCNT | Acid treatment | Fluoroborate bath | Polyacrylic acid | Corrosion resistance | 2010 | [100] |
2.5. Fabrication of Metal/CNT Composites Using Composite Plating by Electroless Deposition
Regarding the number of published articles on metal/CNT composite plating using electroless deposition, those on the Ni-P alloy/CNT is large. In the case of electroless deposition of Ni, phosphorous compounds such as sodium hypophosphite (NaH2PO2) are usually used as the reducing agent and the P derived from the NaH2PO2 is co-deposited with Ni, resulting in Ni-P alloy deposit. Most of the purpose of the fabrication of Ni-P alloy/CNT composites is the improvement of tribological properties. Fabrication conditions in these articles are listed in Table 2.
| Reducing Agent |
|---|
| Surfactant |
|---|
| Remarks |
|---|
| Year | Ref. | ||||||
|---|---|---|---|---|---|---|---|
| Ni-P | MWCNT | Sn2+sensitization + Pd2+activation |
NaH2PO2 | Non | Microstructure, Co-coated CNTs | 2020 | [119] |
| Ni-P | MWCNT | Introduction of -COOH on CNT + Pd2+ | NaH2PO2 | Non | EMI properties, Cotton fabric substrate | 2020 | [120] |
| Ni-P | MWCNT | Sn2+sensitization + Pd2+activation |
NaH2PO2 | Non | Arc discharge synthesized CNTs | 2015 | [121] |
| Ni-P | MWCNT | Sn2+/Pd2+ commercial product | NaH2PO2 | Non | Fe-50Co composites, magnetic properties | 2014 | [122] |
| Au/Ni-P | MWCNT | Sn2+sensitization + Pd2+activation |
NaH2PO2 | Polyacrylic acid (Pre-treatment) |
Improved wettability with molten Al | 2012 | [123] |
| Fe-B/Ni-P | MWCNT | Sn2+sensitization + Pd2+activation |
NaH2PO2, KBH4 | Non | Microwave absorbing properties | 2011 | [124] |
| Ni-P | SWCNT | Sn2+sensitization + Pd2+activation |
NaH2PO2 | Non | Microstructure of Ni-layer | 2011 | [125] |
| Ni-B | MWCNT | Sn2+sensitization + Pd2+activation |
(CH3)2NH·BH3 | Polyacrylic acid (Pre-treatment) |
Graphitized MWCNTs Heat treatment |
2011 | [126] |
| Ni | MWCNT | Sn2+sensitization + Pd2+activation |
N2H4 | Polyacrylic acid (Pre-treatment) |
Graphitized MWCNTs Magnetic properties |
2010 | [127] |
| Ni-P | MWCNT | K2Cr2O7+H2SO4 Sn2+sensitization + Pd2+activation |
NaH2PO2 | Non | Microwave absorbing properties, Ni-N alloy | 2008 | [128] |
| Ni-P | MWCNT | HNO3 Sn2+sensitization + Pd2+activation |
NaH2PO2 | Diallyl-dimethylammonium chloride | Graphitized MWCNTs | 2005 | [129] |
| Ni-P | MWCNT | Sn2+sensitization + Pd2+activation |
NaH2PO2 | Polyacrylic acid (Pre-treatment) |
Graphitized MWCNTs | 2004 | [130] |
| Ni-P | MWCNT | Sn2+sensitization + Pd2+activation |
NaH2PO2 | Non | Continuous Ni-layer | 2002 | [131] |
| Ni-P | MWCNT | Mixed Pd2+/Sn2+ | NaH2PO2 | Non | Pd-coated CNTs | 1999 | [132] |
| Ni-P | MWCNT | Sn2+sensitization + Pd2+activation |
NaH2PO2 | Non | Magnetic property | 1997 | [133] |
| Al | MWCNT | Sn2+/Pd2+ commercial product | LiAlH4 | Non | Non-aqueous bath: AlCl3-urea | 2020 | [134] |
| Ag | MWCNT | H2SO4 + HNO3 Sn2+sensitization + Pd2+activation |
HCHO | Non | Interfacial adhesion of composites | 2004 | [135] |
| Cu | MWCNT | Sulphoric acid + HNO3 Sn2+sensitization + Cu2+activation |
HCHO | Non | Electrical and mechanical properties | 2009 | [136] |
| Cu | MWCNT | HNO3 Sn2+sensitization + Pd2+activation HNO3 |
CHOCOOH | Diallyl-dimethylammonium chloride | Graphitized MWCNTs | 2004 | [137] |
| Co-P | MWCNT | K2Cr2O7+H2SO4 Sn2+sensitization + Pd2+activation |
NaH2PO2 | Non | Heat-treatment | 2000 | [138] |
| Metal | CNT | Pre-Treatment of CNT | Reducing Agent | Surfactant | Remarks | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Ni-P | MWCNT | Non | NaH2PO2 | Sodium lauryl sulfate | Tribological properties, Corrosion resistance |
2021 | [101] |
| Ni-P | MWCNT | Ball milling | NaH2PO2 | Cetyltrimethylammonium bromide | Tribological properties | 2012 | [102] |
| Ni-P | MWCNT | Ball milling, Chemical treatment |
NaH2PO2 | Commercial product | Tribological properties, Corrosion resistance |
2012 | [103] |
| Ni-P | MWCNT | Chemical treatment Ball milling | NaH2PO2 | Sodium lauryl sulfate | Mechanical attrition, Tribological properties | 2012 | [104] |
| Ni-P | MWCNT | HNO3 | Commercial product | Commercial product | Substrate: Mg powder | 2011 | [105] |
| Ni-P | MWCNT | Non | NaH2PO2 | Stearyltrimethylammonium chloride | Substrate: ABS resin Tribological properties |
2011 | [106] |
| Ni-P | MWCNT | Non | NaH2PO2 | Stearyltrimethylammonium chloride | Various P content, Tribological properties |
2010 | [107] |
| Ni-P | MWCNT | Chemical treatment | NaH2PO2 | Unknown | Effects on solder joint | 2009 | [108] |
| Ni-P | MWCNT | Chemical treatment | NaH2PO2 | Cetyltrimethylammonium bromide | Tribological properties | 2009 | [109] |
| Ni-P | MWCNT | Chemical treatment | NaH2PO2 | unknown | Tribological properties | 2006 | [110] |
| Ni-P | MWCNT | Ball milling | NaH2PO2 | Compound name is unknown | Hardness, Corrosion resistance |
2005 | [111] |
| Ni-P | SWCNT | Heat treatment | NaH2PO2 | Compound name is unknown | Tribological properties | 2004 | [112] |
| Ni-P | MWCNT | Ball milling | NaH2PO2 | Cetyltrimethylammonium bromide | Tribological properties | 2003 | [113] |
| Ni-P | MWCNT | Ball milling | NaH2PO2 | Cetyltrimethylammonium bromide | Tribological properties | 2003 | [114] |
| Ni-P | MWCNT | Ball milling | NaH2PO2 | Cetyltrimethylammonium bromide | Tribological properties | 2002 | [115] |
| Cu | SWCNT | Non | CHOCOOH | Sodium lauryl sulfate Hydroxypropylcellulose |
Mechanical disintegration, | 2016 | [116] |
| Cu | MWCNT | Non | CHOCOOH | Sodium lauryl sulfate Hydroxypropylcellulose |
Various CNTs Tribological properties |
2014 | [117] |
| Co-P | MWCNT | Non | NaH2PO2 | Non | Magnetic properties | 2016 | [118] |
3. Metal-Coated CNTs by Electroless Deposition
3.1. Fabrication Process
A fabrication process of metal-coated CNTs by an autocatalytic electroless deposition is schematically showed in Figure 13. Even in the case of electroless deposition, homogeneous dispersion of CNTs in the plating bath is important. The introduction of functional groups on the surface of CNTs likely effective to increase deposition sites, resulting in CNTs coated by metal films and not metal particles.
3.2. Metal-Coated CNTs
Fabrication conditions in these articles are listed in Table 3.
| Metal | CNT | Pre-Treatment of CNT |
|---|
4. Metal/CNT Composites by Electrodeposition Using CNT Templates (Sheet, Yarn)
CNT templates, such as CNT sheets [147,148,149,150][139][140][141][142] and CNT yarns or fibers [151[143][144][145][146],152,153,154], have been developed and their various practical applications have been researched. Although a single CNT has a high electrical conductivity, electrical conductivities of those templates are far less than metals such as Cu, due to the contact resistance between each CNT of which they consist. Therefore, metallization of the CNT templates is a promising process to give them enough electrical conductivity. On the contrary, CNTs have strong anisotropy in electrical and thermal properties [155][147]. Therefore, the orientation of CNTs which make up the templates is also important in order to achieve the expected properties of metal/CNT composites. Fabrication conditions in these articles are listed in Table 4.
| CNT Template | Feature of CNT Template |
Metal | Plating Bath | Remarks | Year | Ref. |
|---|---|---|---|---|---|---|
| MWCNT film | Super-aligned | Cu, Ni | Acid sulfuric bath + glucose Dull Watts Bath |
Improved mechanical and electrical properties | 2019 | [148] |
| MWCNT film | Super-aligned | Ni | Dull Watts Bath | Improved mechanical properties | 2019 | [149] |
| SWCNT paper (Bucky paper) |
Orientation: in-plane direction | Cu | Acid sulfate bath + polyethylene glycol + Cl− + bis(3-sulfopropyl) disulfide + Janus green B | One-step electrodeposition by a combination of additives | 2017 | [150] |
| MWCNT paper | Super-aligned | Cu | Acid sulfuric bath + glucose + polyethylene glycol + Cl− Alkaline bath (EDTA, Citrate) |
Electrical conductivity | 2017 | [151] |
| MWCNT film | Super-aligned | Cu | Acid sulfuric bath + glucose | Improved mechanical properties | 2016 | [152] |
| MWCNT film | Super-aligned | Cu | Acid sulfuric bath + glucose | Improved mechanical properties | 2015 | [153] |
| SWCNT yarn | Straight | Cu | Acid sulfate bath | Graphen growth on the surface of electrodeposited Cu | 2021 | [154] |
| MWCNT yarn | Twisted | Cu | Acid sulfate bath + polyethylene glycol + Cl− + bis(3-sulfopropyl) disulfide + Janus green B | One-step electrodeposition by a combination of additives | 2020 | [155] |
| CNT yarn | Straight | Cu | Acid sulfate bath | Superior current carrying capacity | 2018 | [156] |
| MWCNT yarn | Twisted | Cu | (CH3COO)2 + CH3CN Acid sulfuric bath |
Effect of CNT yarn density | 2018 | [157] |
| MWCNT yarn | Twisted | Cu | Cu (CH3COO)2 + CH3CN Acid sulfuric bath |
Two-step electrodeposition Uniform composite wire |
2017 | [158] |
| MWCNT yarn | Twisted | Cu | (CH3COO)2 + CH3CN Acid sulfuric bath |
Two-step electrodeposition Electrical properties, Solderability, |
2017 | [159] |
| MWCNT yarn | Straight | Cu | Acid sulfuric bath | Electrodeposition of Cu interior of CNT yarn | 2016 | [160] |
| MWCNT yarn | Twisted | Ag, Pt | KNO3+AgNO3 H2SO4 + H2Pt6Cl6 |
Improved tensile strength and electrical conductivity | 2013 | [161] |
| MWCNT yarn | Twisted | Cu | Acid sulfuric bath + octyl phenyl poly (ethylene gylcol) ether | Continuous process: fiber spinning, anodization, electrodeposition | 2011 | [162] |
| MWCNT yarn | Twisted | Au, Pd, Pt, Cu, Ag, Ni | Metal salt solution | Self-fueled electrodeposition Improved electrical conductivity |
2010 | [163] |
5. Conclusions
References
- Oberlin, A.; Endo, M.; Koyama, T. Filamentous growth of carbon through benzene decomposition. J. Cryst. Growth 1976, 32, 335–349.
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58.
- Fink, C.G.; Prince, J.D. The codeposition of copper and graphite. Trans. Am. Electrochem. Soc. 1928, 54, 315–321.
- Guglielmi, N. Kinetics of the Deposition of Inert Particles from Electrolytic Baths. J. Electrochem. Soc. 1972, 119, 1009–1012.
- Buelens, C.; Celis, J.P.; Roos, J.R. Electrochemical aspect of the codeposition of gold and copper with inert particles. J. Appl. Electrochem. 1983, 13, 541–548.
- Celis, J.P.; Roos, J.R.; Buelens, C. A mathematical model for the electrolytic codeposition of particles with a metallic matrix. J. Electrochem. Soc. 1987, 134, 1402–1408.
- Fransaer, J.; Celis, J.P.; Roos, J.R. Analysis of the electrolytic codeposition of non-brownian particles with metals. J. Electrochem. Soc. 1992, 139, 413–425.
- Hwang, B.J.; Hawang, C.S. Mechanism of codeposition of silicon carbide with electrolytic cobalt. J. Electrochem. Soc. 1993, 140, 979–984.
- Vigolo, B.; Penicaud, A.; Coulon, C.; Sauder, C.; Pailler, R.; Journet, C.; Bernier, P.; Paulin, P. Macroscopic fibers and ribbons of oriented carbon nanotubes. Science 2000, 290, 1331–1334.
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band gap fluoresence from individual single-walled carbon nanotubes. Science 2002, 297, 593–596.
- Richard, C.; Balavoine, F.; Schultz, P.; Ebbesen, T.W.; Mioskowski, C. Supramolecular self-assembly of lipid derivatives on car bon nanotubes. Science 2003, 300, 775–778.
- Islam, M.F.; Rojas, E.; Bergey, D.M.; Johnson, A.T.; Yodh, A.G. High weight fraction surfactant solubilization of single-wall carbon nanotubes in water. Nano Lett. 2003, 3, 269–273.
- Moore, V.C.; Strano, M.S.; Haroz, E.H.; Hauge, R.H.; Smally, R.E. Individually suspended single-walled carbon nanotubes in various surfactants. Nano Lett. 2003, 3, 1379–1382.
- Jiang, L.; Gao, L.; Sun, J. Production of aqueous colloidal dispersions of carbon nanotubes. J. Colloid Interface Sci. 2003, 260, 89–94.
- Yurekli, K.; Mitchell, C.A.; Krishnamoorti, R. Small-angle neutron scattering from surfactant-assisted aqueous dispersions of carbon nanotubes. J. Am. Chem. Soc. 2004, 126, 9902–9903.
- Hertel, T.; Hagen, A.; Talalaev, V.; Arnold, K.; Hennrich, F.; Kappes, M.; Rosenthal, S.; McBride, J.; Ulbricht, H.; Flahaut, E. Spectroscopy of single—And double-wall carbon nanotubes environments. Nano Lett. 2005, 5, 511–514.
- Steinmetz, J.; Glerup, M.; Paillet, M.; Bernier, P.; Holzinger, M. Production of pure nanotube fibers using a modified wet-spinning method. Carbon 2005, 43, 2397–2429.
- Tan, Y.; Resasco, D.E. Dispersion of single-walled carbon nanotubes of narrow diameter distribution. J. Phys. Chem. B 2005, 109, 14454–14460.
- Grossiord, N.; van der Schoot, P.; Meuldijk, J.; Koning, C.E. Determination of the surface coverage of exfoliated carbon nanotubes by surfactant molecules in aqueous solution. Langmuir 2007, 23, 3646–3653.
- Sun, Z.; Nicolosi, V.; Rickard, D.; Bergin, S.D.; Aherne, D.; Coleman, J.N. Quantitative evaluation of surfactant-stabilized single-walled carbon nanotubes: Dispersion quality and its correlation with zeta potential. J. Phys. Chem. C 2008, 112, 10692–10699.
- Blanch, A.J.; Lenehan, C.E.; Quinton, J.S. Optimizing surfactant concentrations for dispersion of single-walled carbon nanotubes in aqueous solution. J. Phys. Chem. B 2010, 114, 9805–9811.
- Duan, W.H.; Wang, Q.; Collins, F. Dispersion of carbon nanotubes with SDS surfactants: A study from a binding energy perspective. Chem. Sci. 2011, 2, 1407–1413.
- Barisci, J.N.; Tahhan, M.; Wallace, G.G.; Badaire, S.; Vaugien, T.; Maugey, M.; Poulin, P. Properties of carbon nanotube fibers spun from DNA-stabilized dispersions. Adv. Func. Mat. 2004, 14, 133–138.
- Takahashi, T.; Luculescu, C.R.; Uchida, K.; Ishii, T.; Yajima, H. Dispersion behavior and spectroscopic properties of single-walled carbon nanotubes in chitosan acidic aqueous solutions. Chem. Lett. 2005, 34, 1516–1517.
- Yan, Y.; Cui, J.; Potschke, P.; Voit, B. Dispersion of pristine single-walled carbon nanotubes using pyrene-capped polystyrene and its application for preparation of polystyrene matrix composites. Carbon 2010, 48, 2603–2612.
- Suarez, B.; Simonet, B.M.; Cardenas, S.; Valcarcel, M. Separation of carbon nanotubes in aqueous medium by capillary electrophoresis. J. Chromagr. A 2006, 1128, 282–289.
- Esumi, K.; Ishigami, M.; Nakajima, A.; Sawada, K.; Honda, H. Chemical treatment of carbon nanotubes. Carbon 1995, 33, 279–281.
- Chen, Q.; Dai, L.; Gao, M.; Huang, S.; Mau, A. Plasma activation of carbon nanotubes for chemical modification. J. Phys. Chem. B 2001, 105, 618–622.
- Jiang, L.; Gao, L. Modified carbon nanotubes: An effective way to selective attachment of gold nanoparticles. Carbon 2003, 41, 2923–2929.
- Arai, S.; Endo, M.; Norio, K. Ni-deposited multi-walled carbon nanotubes by electrodepositon. Carbon 2004, 42, 641–644.
- Arai, S.; Endo, M. Carbon nanofiber-copper composite powder prepared by electrodeposition. Electrochem. Commun. 2003, 5, 797–799.
- Jyotheender, K.S.; Gupta, A.; Srivastava, C. Grain boundary engineering in Ni-carbon nanotube composite coatings and its effect on the corrosion behavior of the coatings. Materialia 2020, 9, 100617.
- Prasannakumar, R.S.; Chukwuike, V.I.; Bhakyaraj, K.; Mohan, S.; Barik, R.C. Electrochemical and hydrodynamic flow characterization of corrosion protection of nickel/multiwalled carbon nanotubes composite coating. Appl. Surf. Sci. 2020, 507, 145073.
- Wang, Y.; Chen, J. Preparation and characterization of polydopamine-modified Ni/carbon nanotubes friction composite coating. Coatings 2019, 9, 596.
- Liu, D.G.; Sun, J.; Gui, Z.X.; Song, K.J.; Luo, L.M.; Wu, Y.C. Super-low friction nickel based carbon nanotube composite coating electro-deposited from eutectic solvents. Diam. Relat. Mater. 2017, 74, 229–232.
- Suzuki, T.; Konno, T. Improvement in tool life of electroplated diamond tools by Ni-based carbon nanotube composite coatings. Precis. Eng. 2014, 38, 659–665.
- Yang, Y.J. Morphological and compositional engineering of Ni/carbon nanotube composite film via a novel cyclic voltammetric route. Bull. Mater. Sci. 2012, 35, 513–517.
- Kim, S.K.; Oh, T.S. Electrodeposition behavior and characteristics of Ni-carbon nanotube composite coatings. Trans. Nonferrous Met. Soc. China 2011, 21, s68–s72.
- Martis, P.M.; Dilimon, V.S.; Delhalle, J.; Mekhalif, Z. Electro-generated nickel/carbon nanotube composites in ionic liquid. Electrochim. Acta 2010, 55, 5407–5410.
- Arai, S.; Fujimori, A.; Murai, M.; Endo, M. Excellent solid lubrication of electrodeposited nickel-multiwalled carbon nanotube composite films. Mater. Lett. 2008, 62, 3545–3548.
- Guo, C.; Zuo, Y.; Zhao, X.; Zhao, J.; Xiong, J. Effects of surfactants on electrodeposition of nickel-carbon nanotubes composite coatings. Surf. Coat. Technol. 2008, 202, 3385–3390.
- Guo, C.; Zuo, Y.; Zhao, X.; Zhao, J.; Xiong, J. The effects of electrodeposition current density on properties of Ni-CNTs composite coatings. Surf. Coat. Technol. 2008, 202, 3246–3250.
- Jeon, Y.S.; Byun, J.Y.; Oh, T.S. Electrodeposition and mechanical properties of Ni-carbon nanotube composite coatings. J. Phys. Chem. Solid 2008, 69, 1391–1394.
- Dai, P.Q.; Xu, W.C.; Huang, Q.Y. Mechanical properties and microstructure of nanocrystalline nickel-carbon nanotube composites produced by electrodeposition. Mater. Sci. Eng. A 2008, 483, 172–174.
- Arai, S.; Saito, T.; Endo, M. Low-internal-stress nickel multiwalled carbon nanotube composite electrodeposited from a sulfmate bath. J. Electrochem. Soc. 2007, 154, D530–D533.
- Guo, C.; Zuo, Y.; Zhao, X.; Zhao, J.; Xiong, J. The effects of pulse-reverse parameter on the properties of Ni-carbon nanotube composite coatings. Surf. Coat. Technol. 2007, 201, 9491–9496.
- Arai, S.; Endo, M.; Sato, T.; Koide, A. Fabrication of nickel-multiwalled carbon nanotube composite films with excellent thermal conductivity by an electrodeposition technique. Electrochem. Solid State Lett. 2006, 9, C131–C133.
- Wang, F.; Arai, S.; Endo, M. Preparation of nickel-carbon nanofiber composites by a pulse-reverse electrodeposition process. Electrochem. Commun. 2005, 7, 674–678.
- Chen, X.H.; Chen, C.S.; Xiao, H.N.; Cheng, F.Q.; Zhang, G.; Yi, G.J. Corrosion behavior of carbon nanotube-Ni composite coating. Surf. Coat. Technol. 2005, 191, 351–356.
- Chen, X.H.; Cheng, F.Q.; Li, S.L.; Zhou, L.P.; Li, D.Y. Electrodeposited nickel composites containing carbon nanotubes. Surf. Coat. Technol. 2002, 155, 274–278.
- Chen, X.H.; Peng, J.C.; Li, X.Q.; Deng, F.M.; Wang, J.X.; Li, W.Z. Tribological behavior of carbon nanotubes-reinforced nickel matrix composite coatings. J. Mater. Sci. Lett. 2001, 20, 2057–2060.
- Arora, S.; Kumari, N.; Srivastava, C. Microstructure and corrosion behavior of NiCo-carbon nanotube composite coatings. J. Alloy. Comp. 2019, 801, 449–459.
- Suzuki, Y.; Arai, S.; Endo, M. Electrodeposition of Ni-P alloy-multiwalled carbon nanotube composite films. J. Electrochem. Soc. 2010, 157, D50–D53.
- Shi, L.; Sun, C.F.; Gao, P.; Zhou, F.; Liu, W.M. Electrodeposition and characterization of Ni-Co-carbon manotubes composite coatings. Surf. Coat. Technol. 2006, 200, 4870–4875.
- Shi, Y.L.; Yang, Z.; Xu, H.; Li, M.K.; Li, H.L. Preparation of electroplated Ni-P-ultrafine diamond, Ni-P-carbon nanotubes composite coatings and their corrosion properties. J. Mater. Sci. 2004, 39, 5809–5815.
- Aliyu, A.; Srivastava, C. Corrosion between growth texture, crystallite size, lattice strain and corrosion behavior of copper-carbon nanotube composite coatings. Surf. Coat. Technol. 2021, 405, 126596.
- Li, D.; Xue, J.; Zuo, T.; Gao, Z.; Xiao, L.; Han, L.; Li, S.; Yang, Y. Copper/functionalized-carbon nanotubes composite films with ultrahigh electrical conductivity prepared by pulse reverse electrodeposition. J. Mater. Sci. Mater. Electron. 2020, 31, 14184–14191.
- Wang, M.; Yang, X.; Tao, J.; Bu, Y.; Liu, Y.; Pu, Z.; Yi, J. Achieving high ductility in layered carbon nanotube/copper composite prepared by composite electrodeposition. Diam. Relat. Mater. 2020, 108, 107992.
- Shimizu, M.; Ogasawara, T.; Ohnuki, T.; Arai, S. Multi-layered copper foil reinforced by co-deposition of single-walled carbon nanotube based on electroplating technique. Mater. Lett. 2020, 261, 126993.
- Raja, P.M.; Esquenazi, G.L.; Gowenlock, C.E.; Jones, D.R.; Li, J.; Brinson, B.; Barron, A.R. Electrodeposition of Cu-SWCNT composites. C 2019, 5, 38.
- Ning, D.; Zhang, A.; Wu, H. Enhanced wear performance of Cu-carbon nanotubes composite coatings prepared by jet electrodeposition. Materials 2019, 12, 392.
- Shimizu, M.; Ohnuki, T.; Ogasawara, T.; Banno, T.; Arai, S. Electrodeposited Cu/MWCNT composite film: A potential current collector of silicon-based negative-electrodes for Li-ion batteries. RSC Adv. 2019, 38, 21939–21945.
- Fu, S.; Chen, X.; Liu, P.; Liu, W.; Liu, P.; Zhang, K.; Chen, H. Electrodeposition and properties of composites consisting of carbon nanotubes and copper. J. Mater. Eng. Perform. 2018, 27, 5511–5517.
- Chen, X.; Tao, J.; Yi, J.; Li, C.; Bao, R.; Liu, Y.; You, X.; Tan, S. Balancing the strength and ductility of carbon nanotubes reinforced copper matrix composites with microlaminated structure and interdiffusion. Mater. Sci. Eng. A 2018, 712, 790–793.
- Wang, Z.; Cai, X.; Yang, C.; Zhou, L. An electrodeposition approach to obtaining carbon nanotubes embedded copper powders for the synthesis of copper matrix composites. J. Alloy. Comp. 2018, 735, 1357–1362.
- Wang, Z.; Cai, X.; Yang, C.; Zhou, L. Improving strength and high electrical conductivity of multi-walled carbon nanotubes/copper composites fabricated by electrodeposition and powder metallurgy. J. Alloy. Comp. 2018, 735, 905–913.
- Zheng, L.; Sun, J.; Chen, Q. Carbon nanotubes reinforced copper composite with uniform CNT distribution and high yield of fabrication. Micro Nano Lett. 2017, 12, 722–725.
- Feng, Y.; McGuire, G.E.; Shenderova, O.A.; Ke, H.; Burkett, S.L. Fabrication of copper/carbon nanotube composite thin films by periodic pulse reverse electroplating using nanodiamond as a dispersing agent. Thin Solid Film. 2016, 615, 116–121.
- Arai, S.; Fukuoka, R. A carbon nanotube-reinforced noble tin anode structure for lithium-ion batteries. J. Appl. Electrochem. 2016, 46, 331–338.
- Arai, S.; Kato, A. Mechanism for codeposition of multiwalled carbon nanotubes with copper from acid copper sulfate bath. J. Electrochem. Soc. 2013, 160, D380–D385.
- Qin, X.X.; Liu, J.J.; Wang, F.; Ji, J. Effect of multi-walled carbon nanotubes as second phase on the copper electrochemical reduction behavior for fabricating their nanostructured composite films. J. Electroanal. Chem. 2011, 651, 233–236.
- Arai, S.; Suwa, Y.; Endo, M. Cu/multiwalled carbon nanotube composite films fabricated by pulse-reverse electrodeposition. J. Electrochem. Soc. 2011, 158, D49–D53.
- Arai, S.; Saito, T.; Endo, M. Cu-MWCNT composite films fabricated by electrodeposition. J. Electrochem. Soc. 2010, 157, D147–D153.
- Arai, S.; Saito, T.; Endo, M. Metal-fixed multiwalled carbon nanotube patterned emitters using photolithography and electrodeposition technique. Electrochem. Solid-State Lett. 2008, 11, D72–D74.
- Chai, G.; Sun, Y.; Jenny Sun, J.; Chen, Q. Mechnical properties of carbon nanotube-copper nanocomposites. J. Micromech. Microeng. 2008, 18, 035013.
- Yang, Y.L.; Wang, Y.D.; Ren, Y.; He, C.S.; Deng, J.N.; Nan, J.; Chen, J.G.; Zuo, L. Single-walled carbon nanotube-reinforced copper composite coatings prepared by electrodeposition under ultrasonic field. Mater. Lett. 2008, 62, 47–50.
- Arai, S.; Endo, M. Various carbon nanofiber-copper composite films prepared by electrodeposition. Electrochem. Commun. 2005, 7, 19–22.
- Arai, S.; Endo, M. Carbon nanofiber-copper composites fabricated by electrodeposition. Electrochem. Solid-State Lett. 2004, 7, C25–C26.
- Jyotheender, K.S.; Srivastava, C. Correlating the five-parameter grain boundary character distribution and corrosion behavior of zinc-carbon nanotube composite coatings. Metall. Mater. Trans. A 2021, 52A, 364–377.
- Tseluikin, V.N.; Strilets, A.A.; Yakovlev, A.V. Electrochemical deposition of zinc-based composite coatings modified with carbon nanotubes form alkaline electrolyte. Prot. Met. Phys. Chem. Surf. 2020, 56, 1186–1189.
- Praveen, B.M.; Venkatesha, T.V.; Naik, Y.A.; Prashantha, K. Corrosion studies of carbon nanotube-Zn composite coating. Surf. Coat. Technol. 2007, 201, 5836–5842.
- Tseluikin, V.N.; Koreshkova, A.A. Electrodeposition of zinc-nickel-carbon nanotubes composite coatings in a reversing mode. Prot. Met. Phys. Chem. Surf. 2016, 52, 1040–1042.
- Tripathi, P.; Katiyar, P.K.; Ramkumar, J.; Balani, K. Synergistic role of carbon nanotube and yttria stabilized zirconia reinforcement on wear and corrosion resistance of Cr-based nano-composite coatings. Surf. Coat. Technol. 2020, 385, 125381.
- Shukla, P.; Awasthi, S.; Ramkumar, J.; Balani, K. Protective trivalent Cr-based electrochemical coatings for gun barrels. J. Alloy. Comp. 2018, 769, 1039–1048.
- Liu, B.; Zhen, Z.; Lin, Y. Mechanical properties of hard Cr-MWNT composite coatings. Surf. Coat. Technol. 2009, 203, 3610–3613.
- Pereira, N.M.; Brincoveanu, O.; Pantazi, A.G.; Pereira, C.M.; Araujo, J.P.; Silva, A.F.; Enachescu, M.; Anicai, L. Electrodeposition of Co and Co composites with carbon nanotubes using choline chloride-based ion liquids. Surf. Coat. Technol. 2017, 324, 451–462.
- Arai, S.; Miyagawa, K. Field emission properties of cobalt/multiwalled carbon nanotube composite films fabricated by electrodeposition. Appl. Surf. Sci. 2013, 280, 957–961.
- Arai, S.; Miyagawa, K. Frictional and wear properties of cobalt/multiwalled carbon nanotube composite films formed by electrodeposition. Surf. Coat. Technol. 2013, 235, 204–211.
- Su, F.; Liu, C.; Guo, J.; Huang, P. Characterization of nanocrystalline Co and Co/MWCNT coatings produced by different electrodeposition techniques. Surf. Coat. Technol. 2013, 217, 94–104.
- Anand, E.E.; Natarajan, S. Effect of carbon nanotubes on corrosion and tribological properties of pulse-electrodeposited Co-W composite coatings. J. Mater. Eng. Perform. 2015, 24, 128–135.
- Arai, S.; Miyagawa, K. Fabrication of Co-W alloy/multiwalled carbon nanotube composite films by electrodeposition for improved frictional properties. ECS J. Solid State Sci. Technol. 2013, 2, M39–M43.
- Fugishige, M.; Wongwiriyapan, W.; Wang, F.; Park, K.C.; Takeuchi, K.; Arai, S.; Endo, M. Gold-carbon nanotube composite plating film deposited using non-cyanide bath. Jpn. J. Appl. Phys. 2009, 48, 070217.
- Brandao, A.T.S.C.; Rosoiu, S.; Costa, R.; Lazar, O.A.; Silva, A.F.; Anicai, L.; Pereira, C.M.; Enachescu, M. Characterization and electrochemical studies of MWCNTs decorated with Ag nanoparticles through pulse reversed current electrodeposition using a deep eutectic solvent for energy storage applications. J. Mater. Res. Technol. 2021, 15, 342–359.
- Arai, S.; Kikuhara, T.; Shimizu, M.; Horita, M. Superior electrical contact characteristics of Ag/CNT composite films formed in a cyanide-free plating bath and tested against corrosion by H2S gas. Mater. Lett. 2021, 303, 130504.
- Arai, S.; Kikuhara, T.; Shimizu, M.; Horita, M. Electrodeposition of Ag/CNT composite films from iodide plating baths. J. Electrochem. Soc. 2020, 167, 122515.
- Fujishige, M.; Sekino, M.; Fujisawa, K.; Morimoto, S.; Takeuchi, K.; Arai, S.; Kawai, A. Electric contact characteristic under low load of silver-carbon nanotube composite plating film corroded using H2S gas. Appl. Phys. Express. 2010, 3, 065801.
- Zhang, Z.; Kitada, A.; Chen, T.; Fukami, K.; Shimizu, M.; Arai, S.; Yao, Z.; Murase, K. Dispersion of multiwalled carbon nanotubes into a diglyme solution, electrodeposition of aluminum-based composite, and improvement of hardness. J. Alloy. Comp. 2020, 816, 152585.
- Yatsushiro, T.; Koura, N.; Nakano, S.; Ui, K.; Takeuchi, K. Electrodeposition of aluminum-carbon nanotube composite from room-temperature molten salt electrolyte. Electrochemistry 2006, 74, 233–236.
- Brandao, A.T.S.C.; Anicai, L.; Lazar, O.A.; Rosoiu, S.; Pantazi, A.; Costa, R.; Enachescu, M.; Pereira, C.M.; Silva, A.F. Electrodeposition of Sn and Sn composites with carbon materials using choline chloride-based ionic liquids. Coatings 2019, 9, 798.
- Hu, Z.; Jie, X.; Lu, G. Corrosion resistance of Pb-Sn composite coatings reinforced by carbon nanotubes. J. Coat. Technol. Res. 2010, 7, 809–814.
- Lopes de Oliveira, M.C.; Correa, O.V.; Pereira da Silva, R.M.; Batista de Lima, N.; Dias de Oliveira, J.T.; Antonio de Oliveira, L.; Antunes, R.A. Structural characterization, global and local electrochemical activity of electroless Ni-P-multiwalled carbon nanotube composite coatings on pipeline steel. Metals 2021, 11, 982.
- Meng, Z.Q.; Li, X.B.; Xiong, Y.J.; Zhan, J. Preparation and tribological performances of Ni-P-multi-walled carbon nanotubes composite coatings. T. Nonferr. Met. Soc. 2012, 22, 2719–2725.
- Alishahi, M.; Monirvaghefi, S.M.; Saatchi, A.; Hosseini, S.M. The effect of carbon nanotubes on the corrosion and tribological behavior of electroless Ni-P-CNT composite coating. Appl. Surf. Sci. 2012, 258, 2439–2446.
- Zhao, G.; Ren, C.; He, Y. Ni-P-multiwalled carbon nanotubes composite coatings prepared by mechanical attrition (MA)-assisted electroless plating. Surf. Coat. Technol. 2012, 206, 2774–2779.
- Firoozbakht, M.; Monirvaghefi, S.M.; Niroumand, B. Electroless composite coating of Ni-P-carbon nanotubes on magnesium powder. J. Alloy. Comp. 2011, 509S, S496–S502.
- Arai, S.; Sato, T.; Endo, M. Fabrication of various electrolessb Ni-P alloy/multiwalled carbon nanotube composite films and their frictional properties. J. Electrochem. Soc. 2010, 157, D570–D576.
- Park, C.L.; Fujishige, M.; Takeuchi, K.; Arai, S.; Morimoto, S.; Endo, M. Inter-collisional cutting of multi-walled carbon nanotubes by high-speed agitation. J. Phys. Chem. Solid 2008, 69, 2481–2486.
- Gu, X.; Chan, Y.C.; Yang, D.; Wu, B.Y. The shearing behavior and microstructure of Sn-4Ag-0.5Cu solder joints on a Ni-P-carbon nanotubes composite coating. J. Alloy. Comp. 2009, 468, 553–557.
- Li, Z.H.; Wang, X.Q.; Wang, M.; Wang, F.F.; Ge, H.L. Preparation and tribological properties of the carbon nanotube-Ni-P composite coating. Tribol. Int. 2006, 39, 953–957.
- Chen, X.H.; Chen, C.S.; Xiao, H.N.; Liu, H.B.; Zhou, L.P.; Li, S.L.; Zhang, G. Dry friction and wear characteristics of nickel/carbon nanotube electroless composite deposits. Tribol. Int. 2006, 39, 22–28.
- Yang, Z.; Xu, H.; Shi, Y.L.; Li, M.K.; Huang, Y.; Li, H.L. The fabrication and corrosion behavior of electroless Ni-P-carbon nanotube composite coatings. Mater. Res. Bull. 2005, 40, 1001–1009.
- Yang, Z.; Xu, H.; Li, M.K.; Shi, Y.L.; Huang, Y.; Li, H.L. Preparation and properties of Ni/P/single-walled carbon nanotubes composite coatings by means of electroless plating. Thin Solid Films 2004, 466, 86–91.
- Chen, W.X.; Tu, J.P.; Wang, L.Y.; Gan, H.Y.; Xu, Z.D.; Zhang, X.B. Tribological application of carbon nanotubes in a metal-based composite coatings and composites. Carbon 2003, 41, 215–222.
- Chen, W.X.; Tu, J.P.; Xu, Z.D.; Chen, W.L.; Zhang, X.B.; Cheng, D.H. Tribological properties of Ni-P-multi-walled carbon nanotubes electroless composite coating. Mater. Lett. 2003, 57, 1256–1260.
- Chen, W.X.; Tu, J.P.; Gan, H.Y.; Xu, Z.D.; Wang, Q.G.; Lee, J.Y.; Liu, Z.L.; Zhang, X.B. Electroless preparation and tribological properties of Ni-P-carbon nanotube composite coatings under lubricated condition. Surf. Coat. Technol. 2002, 160, 68–73.
- Arai, S.; Osaki, T.; Hirota, M.; Uejima, M. Fabrication of copper/single-walled carbon nanotube composite film with homogeneously dispersed nanotubes by electroless deposition. Mater. Today Commun. 2016, 7, 101–107.
- Arai, S.; Kanazawa, T. Electroless deposition of Cu/multiwalled carbon nanotube composite films with improved frictional properties. ECS J. Solid State Sci. Technol. 2014, 3, P201–P206.
- Goel, V.; Anderson, P.; Hall, J.; Robinson, F.; Bohm, S. Electroless Co-P-carbon nanotube composite coating to enhance magnetic properties of grain-oriented electrical steel. J. Magn. Magn. Mater. 2016, 407, 42–45.
- Ergul, E.; Kurt, H.I.; Oduncuohlu, M.; Yilmas, N.F. Electroless nickel-phosphorous and cobalt-phosphorous coatings on multi-walled carbon nanotubes. Mater. Res. Express 2020, 7, 115604.
- Qi, Q.; Wang, Y.; Ding, X.; Wang, W.; Xu, R.; Yu, D. High-electromagnetic-shielding cotton fabric prepared using multiwalled carbon nanotubes/nickel-phosphorous electroless plating. Appl. Orgnometal. Chem. 2020, 34, e5434.
- Jagannatham, M.; Sankaran, S.; Haridoss, P. Electroless nickel plating of arc discharged synthesized carbon nanotubes for metal matrix composites. Appl. Surf. Sci. 2015, 324, 475–481.
- Mani, M.K.; Viola, G.; Reece, M.J.; Hall, J.P.; Evans, S.L. Improvement of interfacial bonding in carbon nanotube reinforced Fe-50Co composites by Ni-P coating: Effect on magnetic and mechanical properties. Mater. Sci. Eng. B 2014, 188, 94–101.
- Arai, S.; Suzuki, Y.; Nakagawa, J.; Yamamoto, T.; Endo, M. Fabrication of metal coated carbon nanotubes by electroless deposition for improved wettability with molten aluminum. Surf. Coat. Technol. 2012, 212, 207–213.
- Park, K.Y.; Han, J.H.; Lee, S.B.; Yi, J.W. Microwave absorbing hybrid composites containing Ni-Fe coated carbon nanofibers prepared by electroless plating. Compos. Part A 2011, 42, 573–578.
- Li, W.; Jin, H.; Hao, Y.; Chen, T.; Dai, J.; Wang, Q. The microstructure of Ni layer on single-walled carbon nanotubes prepared by an electroless coating process. J. Nanomater. 2010, 2011, 348958.
- Arai, S.; Imoto, Y.; Suzuki, Y.; Endo, M. Fabrication of Ni-B alloy coated vapor-grown carbon nanofibers by electroless deposition. Carbon 2011, 49, 1484–1490.
- Arai, S.; Kobayashi, M.; Yamamoto, T.; Endo, M. Pure-nickel-coated multiwalled carbon nanotubes prepared by electroless deposition. Electochem. Solis State Lett. 2010, 13, D94–D96.
- Zhao, D.L.; Li, X.; Shen, Z.M. Microwave absorbing property and complex permittivity and permeability of epoxy composites containing Ni-coated and Ag filled carbon nanotubes. Comp. Sci. Technol. 2008, 68, 2902–2908.
- Wang, F.; Arai, S.; Endo, M. The preparation of multi-walled carbon nanotubes with a Ni-P coating by an electroless deposition process. Carbon 2005, 43, 1716–1721.
- Arai, S.; Endo, M.; Hashizume, S.; Shimojima, Y. Nickel-coated carbon nanofibers prepared by electroless deposition. Electochem. Commun. 2004, 6, 1029–1031.
- Kong, F.Z.; Zhang, X.B.; Xiong, W.Q.; Liu, F.; Huang, W.Z.; Sun, Y.L.; Tu, J.P.; Chen, X.W. Continuous Ni-layer on multiwall carbon nanotubes by an electroless plating method. Surf. Coat. Technol. 2002, 155, 33–36.
- Ang, L.M.; Andy Hor, T.S.; Xu, G.Q.; Tung, C.H.; Zhao, S.; Wang, J.L.S. Electroless plating of metals onto carbon nanotubes activated by a single-step activation method. Chem. Mater. 1999, 11, 2115–2118.
- Li, Q.; Fan, S.; Han, W.; Sun, C.; Liang, W. Coating of carbon nanotube with nickel by electroless plating method. Jpn. J. Appl. Phys. 1997, 36, L501–L503.
- Mohammed, E.; Amal, M.K.E. Development of an AlCl3-urea ionic liquid for the electroless deposition of aluminum on carbon nanotubes. ACS Omega 2020, 5, 5756–5761.
- Feng, Y.; Yuan, H. Electroless plating of carbon nanotubes with silver. J. Mater. Sci. 2004, 39, 3241–3243.
- Daoush, W.M.; Lim, B.K.; Mo, C.B.; Nam, D.H.; Hong, S.H. Electrical and mechanical properties of carbon nanotube reinforced copper nanocomposites fabricated by electroless deposition process. Mater. Sci. Eng. A 2009, 513, 247–253.
- Wang, F.; Arai, S.; Endo, M. Metallization of multi-walled carbon nanotubes with copper by an electroless deposition process. Electrochem. Commun. 2004, 6, 1042–1044.
- Chen, X.; Xia, J.; Peng, J.; Li, W.; Xie, S. Carbon-nanotube metal-matrix composites prepared by electroless plating. Compos. Sci. Technol. 2000, 60, 301–306.
- Rinzler, A.G.; Liu, J.; Dai, H.; Nikolaev, P.; Huffman, C.B.; Rodriguez-Macias, F.J.; Boul, P.J.; Lu, A.H.; Heymnn, D.; Colbert, D.T.; et al. Large-scale purification of single-wall carbon nanotubes: Process, product, and characterization. Appl. Phys. A 1998, 67, 29–37.
- Bahr, J.L.; Yang, J.; Kosynkin, D.V.; Bronikowski, M.J.; Smally, R.E.; Tour, J.M. Functionalization of carbon nanotubes by electrochemical reduction of aryl diazonium salts: A bucky paper electrode. J. Am. Chem. Soc. 2001, 123, 6536–6542.
- Ge, J.J.; Hou, H.; Li, Q.; Graham, M.J.; Greiner, A.; Reneker, D.H.; Harris, F.W.; Cheng, S.Z.D. Assembly of well-aligned multiwalled carbon nanotubes in confined polyacrylonitrile environments; electrospun composite nanofiber sheets. J. Am. Chem. Soc. 2004, 126, 15754–15761.
- Feng, C.; Liu, K.; Wu, J.S.; Liu, L.; Cheng, J.S.; Zhang, Y.; Sun, Y.; Li, Q.; Fan, S.; Jiang, K. Flexible, stretchable, transparent conducting films made from superaligned carbon nanotubes. Adv. Funct. Mater. 2010, 20, 885–891.
- Jiang, K.; Li, Q.; Fan, S. Spinning continuous carbon nanotube yarns. Nature 2002, 419, 801.
- Ko, F.; Gogotsi, Y.; Ali, A.; Naguib, N.; Ye, H.; Yang, G.; Li, C.; Willis, P. Electrospinning of continuous carbon nanotube-filled nanofiber yarns. Adv. Mater. 2003, 15, 1161–1165.
- Zhang, M.; Atkinson, K.R.; Baughman, R.H. Multifunctional carbon nanotube yarns by downsizing an ancient technology. Science 2004, 306, 1358–1361.
- Li, Y.L.; Kinloch, I.A.; Windle, A.H. Direct spinning of carbon nanotube fibers from chemical vapor deposition synthesis. Science 2004, 304, 276–278.
- Endo, M.; Kim, Y.A.; Hayashi, T.; Nishimura, K.; Matsusita, T.; Miyashita, K.; Dresselhaus, M.S. Vapor-grown carbon fibers (VGCFs): Basic properties and their battery application. Carbon 2001, 39, 1287–1297.
- Shuai, J.; Xiong, L.; Hou, Z.; Zhu, L.; Li, W. Nickel-coated super-aligned carbon nanotube reinforced copper composite for improved strength and conductivity. J. Mater. Eng. Perform. 2019, 28, 4393–4402.
- Hou, Z.C.; Xiong, L.Q.; Liu, Y.F.; Zhu, L.; Li, W.Z. Preparation of super-aligned carbon nanotube-reinforced nickel-matrix laminar composites with excellent mechanical properties. Int. J. Miner. Metall. Mater. 2019, 26, 133–141.
- Arai, S.; Kirihata, K.; Shimizu, M.; Ueda, M.; Katada, A.; Uejima, M. Fabrication of copper/single-walled carbon nanotube composites by electrodeposition using free-standing nanotube film. J. Electrochem. Soc. 2017, 164, D922–D929.
- Tao, J.M.; Chen, X.F.; Hong, P.; Yi, J.H. Microstructure and electrical conductivity of laminated Cu/CNT/Cu composites prepared by electrodeposition. J. Alloy. Compos. 2017, 717, 232–239.
- Shuai, J.; Xiong, L.; Zhu, L.; Li, W. Enhanced strength and excellent transport properties of a superaligned carbon nanotubes reinforced copper matrix laminar composite. Compos. Part A 2016, 88, 148–155.
- Jin, Y.J.; Zhu, L.; Xue, W.D.; Li, W.Z. Fabrication of superaligned carbon nanotubes reinforced copper matrix laminar composite by electrodeposition. Trans. Nonferrous. Met. Soc. China 2015, 25, 2994–3001.
- Park, M.; Lee, D.M.; Park, M.; Park, S.; Lee, D.S.; Kim, T.W.; Lee, S.H.; Lee, S.K.; Jeong, H.S.; Hong, B.H.; et al. Performance enhancement of graphene assisted CNT/Cu composites for lightweight electrical cables. Carbon 2021, 179, 53–59.
- Arai, S.; Murakami, I.; Shimizu, M.; Oshigane, A. Fabrication of CNT/Cu composite yarn via single-step electrodeposition. J. Electrochem. Soc. 2020, 167, 102509.
- Rho, H.; Park, M.; Park, M.; Park, J.; Han, J.; Lee, A.; Bae, S.; Kim, T.W.; Ha, J.S.; Kim, S.M.; et al. Metal nanofibrils embedded in long free-standing nanotube fiber with a high critical current density. NPA Asia Mater. 2018, 10, 146–155.
- Sundaram, R.; Yamada, T.; Hata, K.; Sekiguchi, A. The importance of carbon nanotube wire density, structural uniformity, and purity for fabricating homogeneous carbon nanotube-copper wire composites by copper electrodeposition. Jpn. J. Appl. Phys. 2018, 57, 04FP08.
- Sundaram, R.; Yamada, T.; Hata, K.; Sekiguchi, A. The influence of Cu electrodeposition parameters on fabricating structurally uniform CNT-Cu composite wires. Mater. Today Commun. 2017, 13, 119–125.
- Sundaram, R.; Yamada, T.; Hata, K.; Sekiguchi, A. Electrical performance of lightweight CNT-Cu composite wires impacted by surface and internal Cu spatial distribution. Sci. Rep. 2017, 7, 9267.
- Hannula, P.M.; Peltonen, A.; Aromaa, J.; Janas, D.; Lundstrom, M.; Wilson, B.P.; Koziol, K.; Forsen, O. Carbon nanotube-copper composites by electrodeposition on carbon nanotube fibers. Carbon 2016, 107, 281–287.
- Chen, T.; Cai, Z.; Qiu, L.; Li, H.; Ren, J.; Lin, H.; Yang, Z.; Sun, X.; Peng, H. Synthesis of aligned carbon nanotube composite fibers with high performances by electrochemical deposition. J. Mater. Chem. A 2013, 1, 2211–2216.
- Xu, G.; Zhao, J.; Li, S.; Zhang, X.; Yong, Z.; Li, Q. Continuous electrodeposition for lightweight, highly conducting and strong carbon nanotube-copper composite fibers. Nanoscale 2011, 3, 4215–4219.
- Lakshman, K.; Randeniya, K.; Bendavid, A.; Martin, P.J.; Tran, C.D. Composite yarns of multiwalled carbon nanotubes with metallic electrical conductivity. Small 2010, 6, 1806–1811.
