Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Bingyuan Wang and Version 2 by Vivi Li.
Apigenin, a common dietary flavonoid abundantly present in a variety of fruits and vegetables, has promising anticancer properties. As an effector of apigenin in myoblasts, protein arginine methyltransferase 7 (Prmt7) is required for male germ cell development.
- apigenin
- spermatogonial proliferation
- Prmt7
- Akt3
- mouse
1. Introduction
Infertility is a global public health issue and a major clinical concern. It is estimated that 8–12% of couples in reproductive age experience infertility worldwide and half of infertility cases are reportedly due to a male factor [1]. A meta-analysis discovered that sperm counts declined by 50–60% within four decades in industrialized countries. Further systematic review as well as meta-regression analysis showed that both sperm concentration and total sperm count exhibited declined trends within four decades [2][3][4][2,3,4]. The causes of infertility are multifactorial, among which nutrition has a significant impact on men’s reproductive health. For example, diets rich in processed meat, soy foods, potatoes, full-fat dairy and total dairy products, cheese, coffee, alcohol, sugar-sweetened beverages and sweets have been associated with detrimental effects on the quality of semen [5]. Several dietary components and nutrients have been considered as possible determinants of sperm function, fertility, or normal function of the reproductive system [6][7][6,7]. Therefore, identifying the dietary components or nutrients that influence male fertility is of great importance for the preservation of fertility, and this is even more so in those cases where fertility is threatened by, e.g., cancer treatment.
Apigenin has promising anticancer properties. Known chemically as 4′,5,7-trihydroxyflavone, apigenin is a common dietary flavonoid abundantly present in a variety of fruits and vegetables. It is one of the most abundant and most studied flavonoids, with molecular formula C15H10O5 [8]. So far, numerous studies have shown that apigenin has potential antioxidant, anti-inflammatory, antibacterial, antiviral, and anticancer properties. Among these properties, the anticancer roles of apigenin have been studied in more than 20 types of cancer. Results suggested that apigenin had selective cytotoxicity on cancer cells while sparing the normal cells from negative consequences [8][9][10][8,9,10]. Therefore, apigenin has been considered as a possible chemotherapeutic agent for cancer therapy. However, it is not clear if apigenin fights cancer while preserving physiological functions, such as reproduction. Apigenin protected testicular cells from acrylonitrile-induced inflammation and apoptosis [11] and sperm from acrylonitrile-induced subchronic injury [12], thereby suggesting that apigenin might be safe for the male germ cells. However, direct proof is missing. Effects of apigenin on male germ cells and its underlying molecular mechanisms are still unclear, and this impedes progress in the applications of apigenin.
It is important to review the mode of action of apigenin in other tissues, in order to infer how it might operate in male germ cells and design meaningful experiments. The mode of action is not entirely clear, but it involves the protein arginine methyltransferase 7 (Prmt7) in the skeletal muscle based on the fact that apigenin was reported to regulate muscle hypertrophy and myogenic differentiation through Prmt7-mediated pathways [13]. This is a member of the type III protein arginine methyltransferase family and the only member of this family that catalyzes monomethylated arginine residues on substrate proteins [14][15][14,15]. Prmt7 knockout mice showed reduced body weight, increased fat mass, and less skeletal muscle mass, suggesting its function in muscle development and adipogenesis [16][17][16,17]. Similarly, PRMT7 deficient patients exhibited mild intellectual disability, obesity, symmetrical shortening of the digits, posterior metacarpals, and metatarsals, also implying its function in the development of skeletal muscle, neurons, bone, and the adipogenesis [17][18][19][20][21][17,18,19,20,21]. Further studies found that Prmt7 was abundantly expressed in male mouse germ cells, including gonocytes and spermatogonia [22]. Depletion of Prmt7 resulted in a defect of primordial germ cell proliferation during mouse embryonic stage, indicating that Prmt7 is also involved in male germ cell proliferation [23]. It has been reported that the addition of apigenin to mouse diet led to quadriceps muscle weight increase in a dose-dependent manner and the muscle fiber size was also increased. Molecular mechanism analyses revealed that apigenin increased the expression of muscle mass regulator Prmt7, which promoted skeletal muscle hypertrophy and myogenic differentiation through Prmt7-PGC-1α-GPR56 pathway and Prmt7-p38-MyoD pathway [13].
2. Apigenin Hampers the Proliferation of Spermatogonia
To determine the effect on spermatogonial proliferation, different concentrations of apigenin (0, 2.5, 5, 10, 20, or 50 μM) were applied in culture for 48 h. Compared with the control group (0 μM), apigenin treatment with 2.5 μM or 5 μM had no effect, while 10, 20, or 50 μM significantly reduced the proliferation of spermatogonia (p < 0.001, Figure 1A). We therefore selected 0, 5, 10, and 20 μM apigenin treatment to assess the cell cycle progression of spermatogonia. As shown in Figure 1B, compared with the control group, 5 and 10 μM apigenin treatment increased the percentage of spermatogonia in S phase. Interestingly, 20 μM apigenin treatment blocked spermatogonia in G2/M phase. Since the cell cycle is regulated by cyclins and cyclin-dependent kinases (Cdks), we further investigated their expression in 20 μM apigenin-treated spermatogonia, compared with the control group. The results showed that the relative mRNA expression of Ccna2, Ccnb1, Cdk1, and Cdk2 was significantly decreased (Figure 1C). Similarly, the protein levels of these cell cycle regulators were also decreased (Figure 1D,E). These findings account the reduced expression of cell cycle regulators Ccna2, Ccnb1, Cdk1, and Cdk2 for the inhibition of spermatogonial proliferation.
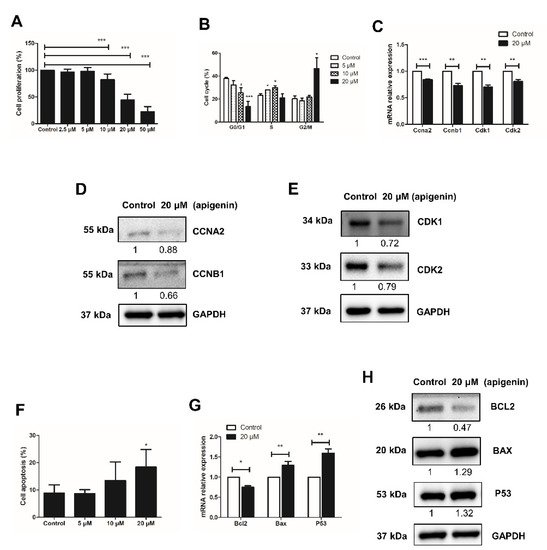

Figure 1. Effects of different concentrations of apigenin treatment on the proliferation and apoptosis of spermatogonia. (A) Cell proliferation was determined by CCK-8 assay after different concentrations of apigenin treatment for 48 h. (B) Cell cycle was analyzed by flow cytometry after applying different concentrations of apigenin treatment for 48 h. (C) Relative mRNA expression of Ccna2, Ccnb1, Cdk1, and Cdk2 examined by RT-qPCR in spermatogonia treated with 0 and 20 μM apigenin for 48 h. (D,E) The protein levels of CCNA2, CCNB1 (D), and CDK1 as well as CDK2 (E) were detected by Western blotting in spermatogonia treated with 0 and 20 μM apigenin for 48 h. (F) Cell apoptosis was analyzed by flow cytometry after applying different concentrations of apigenin for 48 h. (G) Relative mRNA expression of Bcl2, Bax, and P53 examined by RT-qPCR in spermatogonia treated with 0 and 20 μM apigenin for 48 h. (H)The protein levels of BCL2, BAX, P53 were detected by Western blotting in spermatogonia treated with 0 and 20 μM apigenin for 48 h. Data from three independent experiments are expressed as mean ± SEM. * indicates statistical significance. * p < 0.05, ** p < 0.01, and *** p < 0.001.
3. Apigenin Promotes Apoptosis of Spermatogonia
Next to the inhibitory effect of apigenin on the proliferation of spermatogonia, we examined its effect on apoptosis, using Annexin V-PI staining followed by flow cytometry analysis. Compared to control, the apoptosis rate of spermatogonia treated with 5, 10, and 20 μM apigenin increased in a dose-dependent manner: from no effect of 5 μM apigenin, to slightly increased apoptosis rate with 10 μM apigenin, to significantly increased apoptosis with 20 μM apigenin (Figure 1D). Therefore, the expression of apoptosis regulators in spermatogonia was further examined in the presence or absence of 20 μM apigenin. At this concentration Bcl2 expression decreased while Bax and P53 expression increased (Figure 1E,F), indicating that apigenin affected the expression of apoptosis regulators and thereby increased the apoptotic death of spermatogonia.
4. Apigenin Reduces the Expression of Prmt7 and Akt3 in Spermatogonia
The effect of apigenin is probably mediated by multiple downstream effectors. Given the report that Prmt7 and AKT pathways mediated the effect of apigenin on skeletal muscle hypertrophy and myogenic differentiation [13], we examined the effects of apigenin on the expression of Prmt7 and AKT isoforms in spermatogonia. There are three isoforms of AKT, including Akt1, Akt2, and Akt3, among which Akt3 initially was cloned from the rat brain and testis [24]. We found that 20 μM apigenin treatment decreased the expression of Prmt7 on both mRNA and protein level (Figure 2A,B). The mRNA expression of Akt1 was not affected, while the mRNA expression of Akt3 was significantly decreased in spermatogonia treated with 20 μM apigenin (Figure 2B). These results indicated that 20 μM apigenin treatment inhibited the expression of Prmt7 and Akt3 in spermatogonia.
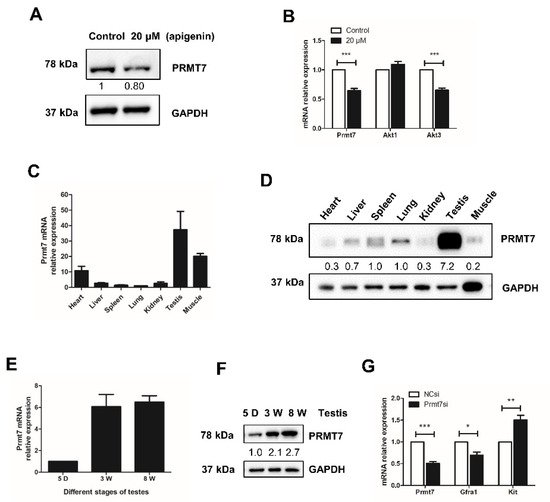

Figure 2. Effects of apigenin treatment on the expression of protein arginine methyltransferase 7 (Prmt7) and Akt3 as well as Prmt7 expression profile in mouse tissues. (A) The protein level of PRMT7 was detected by Western blotting in spermatogonia treated with 0 and 20 μM apigenin for 48 h. (B) Relative mRNA expression of Prmt7, Akt1, and Akt3 was examined by RT-qPCR in spermatogonia treated with 0 and 20 μM apigenin for 48 h. (C) Relative mRNA expression of Prmt7 examined by RT-qPCR in the heart, liver, spleen, lung, kidney, testis, and muscle of 8-week old mice. (D) The protein level of PRMT7 was detected by Western blotting in the heart, liver, spleen, lung, kidney, testis, and muscle of 8-week old mice. (E) Relative mRNA expression of Prmt7 examined by RT-qPCR in the testes of 5-day (5 D), 3-week (3 W), and 8-week (8 W) old mice. (F) The protein level of PRMT7 was detected by Western blotting in the testes of 5 D, 3 W, and 8 W old mice. (G) Relative mRNA expression of Prmt7, Gfra1, and Kit examined by RT-qPCR in negative control siRNA (NCsi)- and Prmt7 siRNA (Prmt7si)-transfected spermatogonia for 48 h. Data from three independent experiments are expressed as mean ± SEM. * indicates statistical significance. * p < 0.05, ** p < 0.01, and *** p < 0.001.
5. Prmt7 Is Abundantly Expressed in Mouse Testis
To further illuminate the molecular mechanism by which apigenin affects Prmt7-mediated spermatogonial proliferation, we profiled the expression of Prmt7 in mouse tissues. Results showed that Prmt7 was extensively expressed in heart, liver, spleen, lung, kidney, testis, and muscle of mouse. Notably, Prmt7 was most abundant in testis (Figure 2C,D). Subsequently, we examined the expression of Prmt7 in different stages of testicular maturation. As shown in Figure 2E,F, Prmt7 expression increased during testicular maturation from 5-day, 3-week, to 8-week, underscoring its potential role in male reproduction. To corroborate this possibility, we examined the markers of spermatogonial stem cells after siRNA-mediated Prmt7 knockdown. Results showed that Prmt7 downregulation was accompanied by decreased expression of the spermatogonial stem cell marker Gfra1, and increased expression of the differentiation marker Kit (Figure 2G). These findings attested to the effect of Prmt7 in spermatogonia.
6. Prmt7 Knockdown Recapitulates the Inhibitory Effects of Apigenin on Spermatogonial Proliferation
To directly test the role of Prmt7 in spermatogonial proliferation, we measured the cell proliferation rate, the cell cycle progression, and the expression of cell cycle regulators after knockdown of Prmt7. After siRNA transfection for 48 h, the cell proliferation rate had declined (Figure 3A). Cell cycle analysis by flow cytometry revealed that the spermatogonia transfected with Prmt7 siRNA were arrested in S phase (Figure 3B). The expression of cell cycle regulators Ccna2 and Cdk2 was decreased on both mRNA and protein level (Figure 3C,D). In addition, we performed Annexin V-PI staining followed by flow cytometry for apoptosis analysis, and examined the expression of apoptosis regulators Bcl2, Bax, and P53 in Prmt7-knockdown spermatogonia. The results showed that downregulation of Prmt7 had no significant effect on the cell apoptosis rate as well as the expression of Bcl2, Bax, and P53, neither on the mRNA nor on the protein level (Figure 3E–G). These findings demonstrated that Prmt7 downregulation arrested spermatogonia in S phase and decreased spermatogonial proliferation rate, which is similar with the effects of apigenin in spermatogonia.
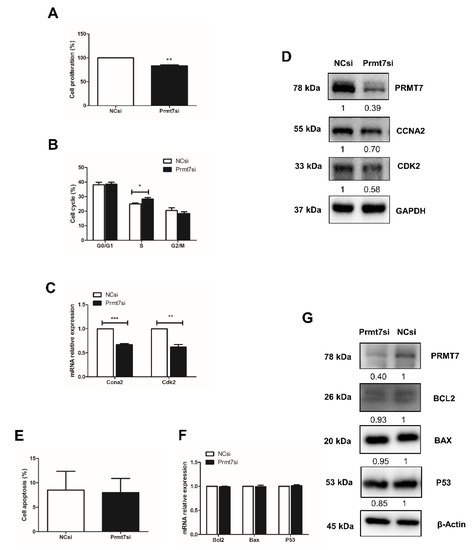

Figure 3. Effects of Prmt7 knockdown on spermatogonial proliferation and apoptosis. (A) Cell proliferation was determined by CCK-8 assay after NC and Prmt7 siRNA transfection into spermatogonia for 48 h. (B) Cell cycle was analyzed by flow cytometry after NC and Prmt7 siRNA transfection into spermatogonia for 48 h. (C) Relative mRNA expression of Ccna2 and Cdk2 examined by RT-qPCR in spermatogonia transfected with NC and Prmt7 siRNA for 48 h. (D) The protein level of PRMT7, CCNA2, and CDK2 detected by Western blotting in spermatogonia transfected with NC and Prmt7 siRNA for 48 h. (E) Cell apoptosis was analyzed by flow cytometry after NC and Prmt7 siRNA transfection into spermatogonia for 48 h. (F) Relative mRNA expression of Bcl2, Bax, and P53 was examined by RT-qPCR in spermatogonia transfected with NC and Prmt7 siRNA for 48 h. (G) The protein levels of PRMT7, BCL2, BAX, and P53 were detected by Western blotting in spermatogonia transfected with NC and Prmt7 siRNA for 48 h. Data from three independent experiments are expressed as mean ± SEM. * indicates statistical significance. * p < 0.05, ** p < 0.01, and *** p < 0.001.
7. Downregulation of Prmt7 Expression Goes beyond This Gene and Perturbs Several Other Genes
To shed light on the possible molecular pathway underlying the similar effects of apigenin treatment and Prmt7 knockdown in spermatogonia, we performed RNA sequencing analysis of Prmt7 siRNA (Prmt7si)-transfected and NC siRNA (NCsi)-transfected spermatogonia, in triplicates. The Prmt7si and the NCsi groups are clearly resolved from each other in the cluster analysis (Figure 4A). In order to identify the differentially expressed genes (DEGs), we used the criteria of adjusted p < 0.05 and |log2 fold change| > = 1. As a result, a total of 287 DEGs were identified in the Prmt7si group compared to NCsi group (Figure 4B), including 256 upregulated DEGs and 31 downregulated DEGs. Next, the 287 DEGs were subjected to the GO functional enrichment analysis. The top 10 GO terms feature detoxification, cellular oxidant detoxification, cellular detoxification (biological process, BP), high-density lipoprotein particle, plasma lipoprotein particle, lipoprotein particle (cellular component, CC), and antioxidant activity, glutathione transferase activity, and low-density lipoprotein particle receptor binding (molecular function, MF), which are shown in Figure 4C. In addition, KEGG enrichment analysis of 287 DEGs revealed six significant pathways, including chemical carcinogenesis, metabolism of xenobiotics by cytochrome P450, drug metabolism-cytochrome P450, steroid hormone biosynthesis, linoleic acid metabolism, and arachidonic acid metabolism (Figure 4D). We checked the RNA sequencing results by RT-qPCR of randomly selected genes. These included 11 downregulated genes (Hap1, Lcor1, Atg9b, Pak1, Rtf1, Rnf112, Nanos1, Sarnp, Tmem62, Kcnh2, Tkfc) and 10 upregulated genes (Clu1, Mmp13, Akr1c18, Slpi, Vnn1, Saa3, Lrsam1, Nqo1, Prg4, Aldh1a1), which were confirmed by RT-qPCR (Figure 4E,F).
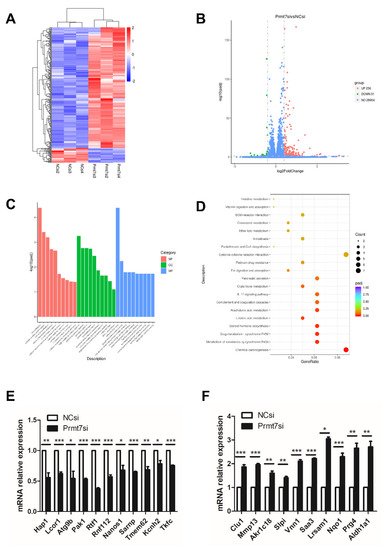

Figure 4. Transcriptome analysis (RNA sequencing) of spermatogonia transfected with NC and Prmt7 siRNA. (A) Clustered heatmap of differentially expressed genes (DEGs) with criteria of adjusted p < 0.05, |log2 fold change| > = 1 was constructed according to the RNA expression level. Two groups with respective three replicates were classified. Blue represents low relative expression of RNAs, and red represents high relative expression of RNAs. (B) The volcano plot of 256 upregulated DEGs and 31 downregulated DEGs in Prmt7si-transfected spermatogonia relative to NCsi-transfected spermatogonia was shown. (C) The top 10 terms of biological process (BP), cellular component (CC), and molecular function (MF) of all DEGs with GO functional enrichment analysis were shown. (D) Top 20 pathways of all DEGs with KEGG pathway enrichment analysis were shown. (E) Verification of randomly selected downregulated DEGs by RT-qPCR. (F) Verification of randomly selected upregulated DEGs by RT-qPCR. Data from three independent experiments are expressed as mean ± SEM. * indicates statistical significance. * p < 0.05, ** p < 0.01, and *** p < 0.001.
2.7. The Possible Functional Pathway Underlying the Regulation of Spermatogonial Proliferation
Among the Prmt7-regulated genes, we also found Akt3, the only AKT isoform identified in downregulated DEGs after Prmt7 knockdown and featured also in the apigenin experiments. Therefore, we hypothesized that apigenin may regulate spermatogonial proliferation through Prmt7/Akt3 pathway. The downregulation of Akt3 (but not Akt2) in Prmt7 siRNA-treated spermatogonia was confirmed by RT-qPCR (Figure 5A), lending further support to the results of the apigenin treatment. To ascertain the robustness of this result, we designed a rescue experiment, using SGC3027 (a potent and selective chemical inhibitor for Prmt7) and SC79 (a unique specific AKT activator). Both Prmt7 siRNA-mediated Prmt7 knockdown and 5 μM SGC3027 treatment-mediated Prmt7 inhibition successfully resulted in decreased PRMT7 protein expression and AKT phosphorylation (Figure 5B,C). We next performed rescue experiments. As shown in Figure 5D, the decreased protein levels of cell cycle regulator CCNA2 and CDK2 in 5 μM SGC3027-treated spermatogonia can be partially rescued by the addition of 10 μM SC79. These results support that apigenin can regulate the proliferation of spermatogonia through the Prmt7/Akt3 pathway.
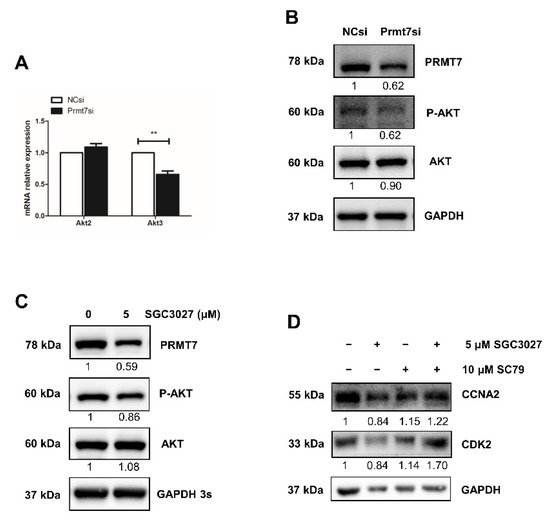

Figure 5. Apigenin/Prmt7/Akt3 pathway analysis in spermatogonial proliferation. (A) Relative mRNA expression of Akt2 and Akt3 was examined by RT-qPCR in spermatogonia transfected with NC and Prmt7 siRNA for 48 h. (B,C) The protein levels of PRMT7 and AKT as well as AKT phosphorylation (P-AKT) were detected by Western blotting in spermatogonia transfected with NC and Prmt7 siRNA (B) or treated with 0 and 5 μM Prmt7 inhibitor SGC3027 for 48 h (C). (D) The protein levels of CCNA2 and CDK2 were detected by Western blotting in spermatogonia treated with 5 μM Prmt7 inhibitor SGC3027 and 10 μM AKT activator SC79 in different combination ways for the rescue assay. Data from three independent experiments are expressed as mean ± SEM. * indicates statistical significance. ** p < 0.01.
