Graphene and graphene nanoribbons hold the promise for improving existing contrast agents as well as drug delivery and biosensing. The entry “Graphene, and Graphene Nanoribbons in Biomedicine” to Encyclopedia is dedicated to applications of graphene and graphene nanoribbons in biomedicine.
- carbon nanotubes
- graphene
- graphene nanoribbons
- biosensing
- bioimaging
- drug delivery
1. Introduction
The entry “Graphene, and Graphene Nanoribbons in Biomedicine” to Encyclopedia is dedicated to applications of graphene and graphene nanoribbons in biomedicine.
2. Applications
Graphene and its derivatives hold the promise for improving existing contrast agents as well as developing completely new probes and agents in biomedical imaging. A wide array of techniques is available to monitor processes in living cells or in tissues or even entire bodies [1]. Radionuclide-based imaging methods are widely employed [2][3][4][5]. Magnetic resonance imaging (MRI) offers a high spatial resolution and is noninvasive. Graphene oxide (GO) on its own is a diamagnetic material and can not be used as a contrast agent for MRI [6]. Graphene does, however, enable photoacoustic imaging. Graphene-based nanomaterials are actively investigated to harness their high near-infrared absorption and conversion to acoustic waves [7][8]. Cancer treatment is clearly in the focus of the development of drug delivery applications of graphene [9][10]. The principal feasibility of pH-controlled drug pickup and delivery by GO for cancer treatment was demonstrated, too [11][12]. More biosensing and imaging applications of graphene were explored in Refs. [13][14][15][16][17].
The finite width of graphene nanoribbons (GNRs) gives, in stark contrast to infinitely extended graphene, rise to lateral quantum confinement, which in turn opens up a semiconducting gap in the band structure. The width-controlled band gap renders GNRs and their derivatives well-suited for optical and near-infrared bioimaging [18][19][20][21]. GNRs also offer a large surface area for crafting functional chemical groups or physisorbed moieties. Oxidized (oGNRs) and reduced graphene nanoribbons (rGNRs) can be prepared in the same way as GO and are envisaged as a unique drug delivery agents in cancer and tumor therapy [22][23]. In Reference [23], mice were injected with 99mTc-labeled and polyethylene glycol-coated (PEGylated) graphene oxide nanoribbons (PL-PEG-GONRs). Single photon emission computed tomography (SPECT)/ computed tomography (CT) images revealed the biodistribution in the mice 30 min after injection. There was also a strong signal in the bladder (Fig. 1a) [23]. The volume-rendering images of 99mTc-labeled PL-PEG-GONRs in mice were obtained at different times after injection (0.25, 0.75, 1.25 and 1.75 h) and showed that a strong signal appeared in the kidney after 1.75 h (Fig. 1b) [23]. The series show the renal clearance of PL-PEG-GONRs in vivo.
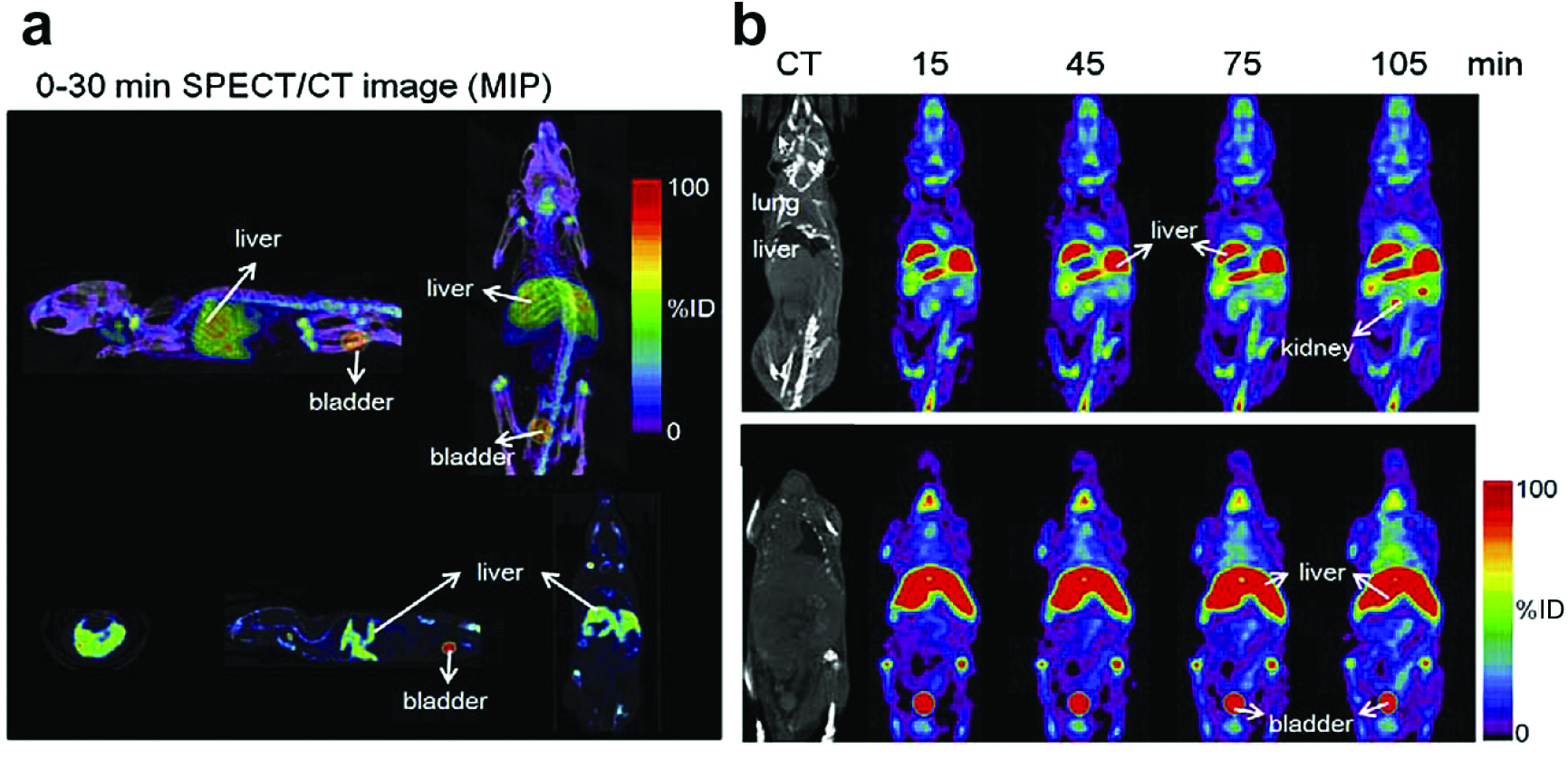
Figure 1. (a) Whole-body SPECT/CT images of 99mTc-labeled PL-PEG-GONRs in mice (from 2 angles) 0.5 h after injection. (b) Whole-body SPECT/CT and volume-rendering images of 99mTc labeled PL-PEG-GONRs in mice (0.25, 0.75, 1.25 and 1.75 h) after injection. Reprinted from [23], Copyright 2014, with permission from Elsevier.
GNRs were also employed for applications in biosensors [24][25][26]. Ultrasensitive targeting of deoxyribonucleic acid by nanoparticle-functionalized GNRs and detection by GNR-based field-effect transistor were shown [27][28].
Next to graphene and graphene nanoribbons, carbon nanotubes are also considered as promising materials for biomedical applications. In particular, endohedrally functionalized single-walled carbon nanotubes [29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75] can be similarily applied in bioimaging, drug delivery and biosensing. The field of medical bioapplications of carbon nanomaterials is actively developed and will in the near future revolutionize our therapeutic and diagnostic capabilities.
References
- Xuesong Li; Weiwei Cai; Jinho An; Seyoung Kim; Junghyo Nah; Dongxing Yang; Richard Piner; Aruna Velamakanni; Inhwa Jung; Emanuel Tutuc; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312-1314, 10.1126/science.1171245.
- Lei Chen; Xiaoyan Zhong; Xuan Yi; Min Huang; Ping Ning; Teng Liu; Cuicui Ge; Zhifang Chai; Zhuang Liu; Kai Yang; et al. Radionuclide 131I labeled reduced graphene oxide for nuclear imaging guided combined radio- and photothermal therapy of cancer. Biomaterials 2015, 66, 21-28, 10.1016/j.biomaterials.2015.06.043.
- Hao Hong; Yin Zhang; Jonathan W Engle; Tapas Nayak; Charles P. Theuer; Robert J. Nickles; Todd Barnhart; Weibo Cai; In vivo targeting and positron emission tomography imaging of tumor vasculature with 66Ga-labeled nano-graphene. Biomaterials 2012, 33, 4147-4156, 10.1016/j.biomaterials.2012.02.031.
- Hao Hong; Kai Yang; Yin Zhang; Jonathan W Engle; Liangzhu Feng; Yunan Yang; Tapas Nayak; Shreya Goel; Jero Bean; Charles P. Theuer; et al. In Vivo Targeting and Imaging of Tumor Vasculature with Radiolabeled, Antibody-Conjugated Nanographene. ACS Nano 2012, 6, 2361-2370, 10.1021/nn204625e.
- Sixiang Shi; Kai Yang; Hao Hong; Feng Chen; Hector Valdovinos; Shreya Goel; Todd Barnhart; Zhuang Liu; Weibo Cai; VEGFR targeting leads to significantly enhanced tumor uptake of nanographene oxide in vivo. Biomaterials 2014, 39, 39-46, 10.1016/j.biomaterials.2014.10.061.
- Zhenyu Gao; Tiancong Ma; Enyu Zhao; Dominic Docter; Wensheng Yang; Roland H. Stauber; Mingyuan Gao; Small is Smarter: Nano MRI Contrast Agents - Advantages and Recent Achievements. Small 2015, 12, 556-576, 10.1002/smll.201502309.
- Jingqin Chen; Chengbo Liu; Guang Zeng; Yujia You; Huina Wang; Xiaojing Gong; Rongqin Zheng; Jeesu Kim; Chulhong Kim; Liang Song; et al. Indocyanine Green Loaded Reduced Graphene Oxide for In Vivo Photoacoustic/Fluorescence Dual-Modality Tumor Imaging. Nanoscale Research Letters 2016, 11, 1-11, 10.1186/s11671-016-1288-x.
- Kai Yang; Lilei Hu; Xingxing Ma; Shuoqi Ye; Liang Cheng; Xiaoze Shi; Changhui Li; Yonggang Li; Zhuang Liu; Multimodal Imaging Guided Photothermal Therapy using Functionalized Graphene Nanosheets Anchored with Magnetic Nanoparticles. Advanced Materials 2012, 24, 1868-1872, 10.1002/adma.201104964.
- Zhuang Liu; Joshua T. Robinson; XiaoMing Sun; Hongjie Dai; PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. Journal of the American Chemical Society 2008, 130, 10876-10877, 10.1021/ja803688x.
- Liming Zhang; Jingguang Xia; Qinghuan Zhao; Liwei Liu; Zhijun Zhang; Functional Graphene Oxide as a Nanocarrier for Controlled Loading and Targeted Delivery of Mixed Anticancer Drugs. Small 2010, 6, 537-544, 10.1002/smll.200901680.
- Mei-Ling Chen; Jia-Wei Liu; Bo Hu; Ming-Li Chen; Jian-Hua Wang; Conjugation of quantum dots with graphene for fluorescence imaging of live cells. The Analyst 2011, 136, 4277-4283, 10.1039/c1an15474e.
- Xiaoying Yang; Xiaoyan Zhang; Zunfeng Liu; Yanfeng Ma; Yi Huang; Yongsheng Chen; High-Efficiency Loading and Controlled Release of Doxorubicin Hydrochloride on Graphene Oxide. The Journal of Physical Chemistry C 2008, 112, 17554-17558, 10.1021/jp806751k.
- Yuan-Pin Huang; Chao-Ming Hung; Yi-Chiang Hsu; Cai-Yan Zhong; Wan-Rou Wang; Chi-Chang Chang; Mon-Juan Lee; Suppression of Breast Cancer Cell Migration by Small Interfering RNA Delivered by Polyethylenimine-Functionalized Graphene Oxide. Nanoscale Research Letters 2016, 11, 1-8, 10.1186/s11671-016-1463-0.
- Yujun Song; Konggang Qu; Chao Zhao; Jinsong Ren; Xiaogang Qu; Graphene Oxide: Intrinsic Peroxidase Catalytic Activity and Its Application to Glucose Detection. Advanced Materials 2010, 22, 2206-2210, 10.1002/adma.200903783.
- Kun Wang; Qian Liu; Qing-Meng Guan; Jun Wu; Henan Li; Jia-Jia Yan; Enhanced direct electrochemistry of glucose oxidase and biosensing for glucose via synergy effect of graphene and CdS nanocrystals. Biosensors and Bioelectronics 2011, 26, 2252-2257, 10.1016/j.bios.2010.09.043.
- Janire Peña-Bahamonde; Hang N. Nguyen; Sofia K. Fanourakis; Debora F. Rodrigues; Recent advances in graphene-based biosensor technology with applications in life sciences. Journal of Nanobiotechnology 2018, 16, 1-17, 10.1186/s12951-018-0400-z.
- Changsheng Shan; Huafeng Yang; Dongxue Han; Qixian Zhang; Ari Ivaska; Li Niu; Electrochemical determination of NADH and ethanol based on ionic liquid-functionalized graphene. Biosensors and Bioelectronics 2010, 25, 1504-1508, 10.1016/j.bios.2009.11.009.
- Ayrat Gizzatov; Vazrik Keshishian; Adem Guven; Ayrat M. Dimiev; Feifei Qu; Raja Muthupillai; Paolo Decuzzi; Robert G. Bryant; James M. Tour; Lon J. Wilson; et al. Enhanced MRI relaxivity of aquated Gd3+ ions by carboxyphenylated water-dispersed graphene nanoribbons. Nanoscale 2014, 6, 3059-3063, 10.1039/c3nr06026h.
- Xinxing Ma; Huiquan Tao; Kai Yang; Liangzhu Feng; Liang Cheng; Xiaoze Shi; Yonggang Li; Liang Guo; Zhuang Liu; A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Research 2012, 5, 199-212, 10.1007/s12274-012-0200-y.
- Omid Akhavan; Elham Ghaderi; Hamed Emamy; Nontoxic concentrations of PEGylated graphene nanoribbons for selective cancer cell imaging and photothermal therapy. Journal of Materials Chemistry 2012, 22, 20626-20633, 10.1039/c2jm34330d.
- Hong Zhao; Ruihua Ding; Xin Zhao; Yiwei Li; Liangliang Qu; Hao Pei; Lara Yildirimer; Zhengwei Wu; Weixia Zhang; Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discovery Today 2017, 22, 1302-1317, 10.1016/j.drudis.2017.04.002.
- Sayan Mullick Chowdhury; Cassandra Surhland; Zina Sanchez; Pankaj Chaudhary; M.A. Suresh Kumar; Stephen Lee; Louis A. Peña; Michael Waring; Balaji Sitharaman; Mamta Naidu; et al. Graphene nanoribbons as a drug delivery agent for lucanthone mediated therapy of glioblastoma multiforme. Nanomedicine: Nanotechnology, Biology and Medicine 2014, 11, 109-118, 10.1016/j.nano.2014.08.001.
- Yu-Jen Lu; Chih-Wen Lin; Hung-Wei Yang; Kun-Ju Lin; Shiaw-Pyng Wey; Chia-Liang Sun; Kuo-Chen Wei; Tzu-Chen Yen; Ching-I Lin; Chen-Chi M. Ma; et al. Biodistribution of PEGylated graphene oxide nanoribbons and their application in cancer chemo-photothermal therapy. Carbon 2014, 74, 83-95, 10.1016/j.carbon.2014.03.007.
- Di Yin; Yang Li; Hang Lin; Baofeng Guo; Yanwei Du; Xin Li; Huijie Jia; Xuejian Zhao; Jun Tang; Ling Zhang; et al. Functional graphene oxide as a plasmid-based Stat3 siRNA carrier inhibits mouse malignant melanoma growth in vivo. Nanotechnology 2013, 24, 105102, 10.1088/0957-4484/24/10/105102.
- A. B. M. Zakaria; Danuta Leszczynska; Electrochemically Prepared Unzipped Single Walled Carbon Nanotubes-MnO2 Nanostructure Composites for Hydrogen Peroxide and Glucose Sensing. Chemosensors 2019, 7, 1, 10.3390/chemosensors7010001.
- L. Jothi; N. Jayakumar; Saravana Kumar Jaganathan; G. Nageswaran; Ultrasensitive and selective non-enzymatic electrochemical glucose sensor based on hybrid material of graphene nanosheets/graphene nanoribbons/nickel nanoparticle. Materials Research Bulletin 2018, 98, 300-307, 10.1016/j.materresbull.2017.10.020.
- Qiumei Feng; Xiaolei Zhao; Yuehua Guo; Mingkai Liu; Po Wang; Stochastic DNA walker for electrochemical biosensing sensitized with gold nanocages@graphene nanoribbons. Biosensors and Bioelectronics 2018, 108, 97-102, 10.1016/j.bios.2018.02.050.
- Morteza Rastgoo; Morteza Fathipour; Interaction of DNA nucleobases with boron, nitrogen, and sulfur doped graphene nano-ribbon for sequencing: An Ab initio study. Applied Surface Science 2019, 492, 634-643, 10.1016/j.apsusc.2019.06.208.
- M. V. Kharlamova; M. M. Brzhezinskay; A. S. Vinogradov; I. P. Suzdalev; Yu. V. Maksimov; V. K. Imshennik; S. V. Novichikhin; A. V. Krestinin; L. V. Yashina; A. V. Lukashin; et al. The formation and properties of one-dimensional FeHal2 (Hal = Cl, Br, I) nanocrystals in channels of single-walled carbon nanotubes. Nanotechnologies in Russia 2009, 4, 634-646, 10.1134/s1995078009090080.
- Andrei A. Eliseev; Marianna V. Kharlamova; Marina V. Chernysheva; Alexey V. Lukashin; Yuri D. Tretyakov; Andrey Kumskov; Nikolay A. Kiselev; Preparation and properties of single-walled nanotubes filled with inorganic compounds. Russian Chemical Reviews 2009, 78, 833-854, 10.1070/rc2009v078n09abeh004077.
- Marianna V. Kharlamova; Andrei Eliseev; Lada Yashina; Dmitrii Petukhov; Chan-Pu Liu; Chen-Yu Wang; Dmitry A. Semenenko; Alexander I. Belogorokhov; Study of the electronic structure of single-walled carbon nanotubes filled with cobalt bromide. JETP Letters 2010, 91, 196-200, 10.1134/s0021364010040089.
- A. Eliseev; L.V. Yashina; M. Brzhezinskaya; M.V. Chernysheva; M.V. Kharlamova; N.I. Verbitsky; A.V. Lukashin; N. Kiselev; A. Kumskov; R.M. Zakalyuhin; et al.J.L. Hutchison Structure and electronic properties of AgX (X=Cl, Br, I)-intercalated single-walled carbon nanotubes. Carbon 2010, 48, 2708-2721, 10.1016/j.carbon.2010.02.037.
- L. V. Yashina; A. A. Eliseev; M. V. Kharlamova; A. A. Volykhov; A. V. Egorov; S. V. Savilov; A. V. Lukashin; R. Püttner; A. I. Belogorokhov; Growth and Characterization of One-Dimensional SnTe Crystals within the Single-Walled Carbon Nanotube Channels. The Journal of Physical Chemistry C 2011, 115, 3578-3586, 10.1021/jp1107087.
- M. V. Kharlamova; L. Yashina; A. Volykhov; J. J. Niu; V. S. Neudachina; M. Brzhezinskaya; T. S. Zyubina; A. I. Belogorokhov; A. Eliseev; Acceptor doping of single-walled carbon nanotubes by encapsulation of zinc halogenides. The European Physical Journal B 2012, 85, 34, 10.1140/epjb/e2011-20457-6.
- M. V. Kharlamova; J. J. Niu; New method of the directional modification of the electronic structure of single-walled carbon nanotubes by filling channels with metallic copper from a liquid phase. JETP Letters 2012, 95, 314-319, 10.1134/s0021364012060057.
- A. Eliseev; L. Yashina; N.I. Verbitskiy; M. Brzhezinskaya; M. Kharlamova; M.V. Chernysheva; A.V. Lukashin; N. Kiselev; A. Kumskov; B. Freitag; et al.A.V. GeneralovA. VinogradovY.V. ZubavichusE. KleimenovM. Nachtegaal Interaction between single walled carbon nanotube and 1D crystal in CuX@SWCNT (X=Cl, Br, I) nanostructures. Carbon 2012, 50, 4021-4039, 10.1016/j.carbon.2012.04.046.
- M. V. Kharlamova; J. J. Niu; Donor doping of single-walled carbon nanotubes by filling of channels with silver. Journal of Experimental and Theoretical Physics 2012, 115, 485-491, 10.1134/s1063776112080092.
- M. V. Kharlamova; Lada Yashina; Andrei Eliseev; Andrey Volykhov; V. S. Neudachina; Maria Brzhezinskaya; T. S. Zyubina; A. V. Lukashin; Yu. D. Tretyakov; Single-walled carbon nanotubes filled with nickel halogenides: Atomic structure and doping effect. physica status solidi (b) 2012, 249, 2328-2332, 10.1002/pssb.201200060.
- M. V. Kharlamova; J. J. Niu; Comparison of metallic silver and copper doping effects on single-walled carbon nanotubes. Applied Physics B 2012, 109, 25-29, 10.1007/s00339-012-7091-3.
- Marianna V. Kharlamova; Markus Sauer; Takeshi Saito; Stefan Krause; Xianjie Liu; Kazuhiro Yanagi; Thomas Pichler; Hidetsugu Shiozawa; Inner tube growth properties and electronic structure of ferrocene-filled large diameter single-walled carbon nanotubes. physica status solidi (b) 2013, 250, 2575-2580, 10.1002/pssb.201300089.
- M. V. Kharlamova; Electronic properties of pristine and modified single-walled carbon nanotubes. Physics-Uspekhi 2013, 56, 1047-1073, 10.3367/ufne.0183.201311a.1145.
- M. V. Kharlamova; Novel approach to tailoring the electronic properties of single-walled carbon nanotubes by the encapsulation of high-melting gallium selenide using a single-step process. JETP Letters 2013, 98, 272-277, 10.1134/s0021364013180069.
- M. V. Kharlamova; L. Yashina; A. V. Lukashin; Comparison of modification of electronic properties of single-walled carbon nanotubes filled with metal halogenide, chalcogenide, and pure metal. Applied Physics B 2013, 112, 297-304, 10.1007/s00339-013-7808-y.
- M. V. Kharlamova; L. V. Yashina; A. V. Lukashin; Charge transfer in single-walled carbon nanotubes filled with cadmium halogenides. Journal of Materials Science 2013, 48, 8412-8419, 10.1007/s10853-013-7653-6.
- M. V. Kharlamova; Comparison of influence of incorporated 3d-, 4d- and 4f-metal chlorides on electronic properties of single-walled carbon nanotubes. Applied Physics B 2013, 111, 725-731, 10.1007/s00339-013-7639-x.
- Christian Kramberger; Marianna V. Kharlamova; Kazuhiro Yanagi; Multifrequency Raman spectroscopy on bulk (11,10) chirality enriched semiconducting single-walled carbon nanotubes. physica status solidi (b) 2014, 251, 2432-2436, 10.1002/pssb.201451182.
- M. V. Kharlamova; Comparative analysis of electronic properties of tin, gallium, and bismuth chalcogenide-filled single-walled carbon nanotubes. Journal of Materials Science 2014, 49, 8402-8411, 10.1007/s10853-014-8550-3.
- Marianna V. Kharlamova; Christian Kramberger; Takeshi Saito; Hidetsugu Shiozawa; Thomas Pichler; In situRaman spectroscopy studies on time-dependent inner tube growth in ferrocene-filled large diameter single-walled carbon nanotubes. physica status solidi (b) 2014, 251, 2394-2400, 10.1002/pssb.201451166.
- M. V. Kharlamova; Andrey Volykhov; Lada Yashina; A. V. Egorov; A. V. Lukashin; Experimental and theoretical studies on the electronic properties of praseodymium chloride-filled single-walled carbon nanotubes. Journal of Materials Science 2015, 50, 5419-5430, 10.1007/s10853-015-9086-x.
- Marianna V. Kharlamova; Christian Kramberger; Markus Sauer; Kazuhiro Yanagi; Thomas Pichler; Comprehensive spectroscopic characterization of high purity metallicity-sorted single-walled carbon nanotubes. physica status solidi (b) 2015, 252, 2512-2518, 10.1002/pssb.201552251.
- Marianna V. Kharlamova; Markus Sauer; Alexander Egorov; Christian Kramberger; Takeshi Saito; Thomas Pichler; Hidetsugu Shiozawa; Temperature-dependent inner tube growth and electronic structure of nickelocene-filled single-walled carbon nanotubes. physica status solidi (b) 2015, 252, 2485-2490, 10.1002/pssb.201552206.
- M. V. Kharlamova; Rare-earth metal halogenide encapsulation-induced modifications in Raman spectra of single-walled carbon nanotubes. Applied Physics A 2014, 118, 27-35, 10.1007/s00339-014-8880-7.
- Marianna V. Kharlamova; Markus Sauer; Takeshi Saito; Yuta Sato; Kazu Suenaga; Thomas Pichler; Hidetsugu Shiozawa; Doping of single-walled carbon nanotubes controlled via chemical transformation of encapsulated nickelocene. Nanoscale 2014, 7, 1383-1391, 10.1039/c4nr05586a.
- Marianna V. Kharlamova; Raman Spectroscopy Study of the Doping Effect of the Encapsulated Iron, Cobalt, and Nickel Bromides on Single-Walled Carbon Nanotubes. Journal of Spectroscopy 2015, 2015, 1-8, 10.1155/2015/653848.
- Marianna V. Kharlamova; Advances in tailoring the electronic properties of single-walled carbon nanotubes. Progress in Materials Science 2016, 77, 125-211, 10.1016/j.pmatsci.2015.09.001.
- Marianna V. Kharlamova; Christian Kramberger; Thomas Pichler; Semiconducting response in single-walled carbon nanotubes filled with cadmium chloride. physica status solidi (b) 2016, 253, 2433-2439, 10.1002/pssb.201600300.
- M. V. Kharlamova; Electronic properties of single-walled carbon nanotubes filled with manganese halogenides. Applied Physics A 2016, 122, 791, 10.1007/s00339-016-0335-x.
- M. V. Kharlamova; Christian Kramberger; Takeshi Saito; Hidetsugu Shiozawa; Thomas Pichler; Growth dynamics of inner tubes inside cobaltocene-filled single-walled carbon nanotubes. Applied Physics A 2016, 122, 749, 10.1007/s00339-016-0282-6.
- M. V. Kharlamova; C. Kramberger; A. Mittelberger; Raman spectroscopy study of the doping effect of the encapsulated terbium halogenides on single-walled carbon nanotubes. Applied Physics A 2017, 123, 239, 10.1007/s00339-017-0873-x.
- Marianna V. Kharlamova; Investigation of growth dynamics of carbon nanotubes. Beilstein Journal of Nanotechnology 2017, 8, 826-856, 10.3762/bjnano.8.85.
- Marianna V. Kharlamova; Christian Kramberger; Takeshi Saito; Yuta Sato; Kazu Suenaga; Thomas Pichler; Hidetsugu Shiozawa; Chirality-dependent growth of single-wall carbon nanotubes as revealed inside nano-test tubes. Nanoscale 2017, 9, 7998-8006, 10.1039/c7nr01846k.
- Marianna V. Kharlamova; Christian Kramberger; Kazuhiro Yanagi; Markus Sauer; Takeshi Saito; Thomas Pichler; Separation of Nickelocene-Filled Single-Walled Carbon Nanotubes by Conductivity Type and Diameter. physica status solidi (b) 2017, 254, 1700178, 10.1002/pssb.201700178.
- M. V. Kharlamova; C. Kramberger; M. Sauer; K. Yanagi; T. Saito; T. Pichler; Inner tube growth and electronic properties of metallicity-sorted nickelocene-filled semiconducting single-walled carbon nanotubes. Applied Physics A 2018, 124, 247, 10.1007/s00339-018-1679-1.
- M. V. Kharlamova; C. Kramberger; A. Mittelberger; K. Yanagi; T. Pichler; D. Eder; Silver Chloride Encapsulation-Induced Modifications of Raman Modes of Metallicity-Sorted Semiconducting Single-Walled Carbon Nanotubes. Journal of Spectroscopy 2018, 2018, 1-9, 10.1155/2018/5987428.
- Marianna V. Kharlamova; Christian Kramberger; Yuta Sato; Takeshi Saito; Kazu Suenaga; Thomas Pichler; Hidetsugu Shiozawa; Chiral vector and metal catalyst-dependent growth kinetics of single-wall carbon nanotubes. Carbon 2018, 133, 283-292, 10.1016/j.carbon.2018.03.046.
- Marianna V. Kharlamova; Christian Kramberger; Oleg Domanov; Andreas Mittelberger; Kazuhiro Yanagi; Thomas Pichler; Dominik Eder; Fermi level engineering of metallicity-sorted metallic single-walled carbon nanotubes by encapsulation of few-atom-thick crystals of silver chloride. Journal of Materials Science 2018, 53, 13018-13029, 10.1007/s10853-018-2575-y.
- Marianna V. Kharlamova; Christian Kramberger; Oleg Domanov; Andreas Mittelberger; Takeshi Saito; Kazuhiro Yanagi; Thomas Pichler; Dominik Eder; Comparison of Doping Levels of Single‐Walled Carbon Nanotubes Synthesized by Arc‐Discharge and Chemical Vapor Deposition Methods by Encapsulated Silver Chloride. physica status solidi (b) 2018, 255, 1800178, 10.1002/pssb.201800178.
- Marianna V. Kharlamova; Christian Kramberger; Paolo Rudatis; Thomas Pichler; Dominik Eder; Revealing the doping effect of encapsulated lead halogenides on single-walled carbon nanotubes. Applied Physics A 2019, 125, 320, 10.1007/s00339-019-2626-5.
- Marianna V. Kharlamova; Christian Kramberger; Paolo Rudatis; Kazuhiro Yanagi; Dominik Eder; Characterization of the Electronic Properties of Single‐Walled Carbon Nanotubes Filled with an Electron Donor—Rubidium Iodide: Multifrequency Raman and X‐ray Photoelectron Spectroscopy Studies. physica status solidi (b) 2019, 256, 1900209, 10.1002/pssb.201900209.
- Marianna V. Kharlamova; Christian Kramberger; Takeshi Saito; Thomas Pichler; Diameter and metal-dependent growth properties of inner tubes inside metallocene-filled single-walled carbon nanotubes. Fullerenes, Nanotubes and Carbon Nanostructures 2019, 28, 20-26, 10.1080/1536383x.2019.1671360.
- Marianna V. Kharlamova; Nickelocene-Filled Purely Metallic Single-Walled Carbon Nanotubes: Sorting and Tuning the Electronic Properties. Nanomaterials 2021, 11, 2500, 10.3390/nano11102500.
- Marianna V. Kharlamova; Christian Kramberger; Metal Cluster Size-Dependent Activation Energies of Growth of Single-Chirality Single-Walled Carbon Nanotubes inside Metallocene-Filled Single-Walled Carbon Nanotubes. Nanomaterials 2021, 11, 2649, 10.3390/nano11102649.
- Maria G. Burdanova; Marianna V. Kharlamova; Christian Kramberger; Maxim P. Nikitin; Applications of Pristine and Functionalized Carbon Nanotubes, Graphene, and Graphene Nanoribbons in Biomedicine. Nanomaterials 2021, 11, 3020, 10.3390/nano11113020.
- Marianna V. Kharlamova; Christian Kramberger; Temperature-Dependent Growth of 36 Inner Nanotubes inside Nickelocene, Cobaltocene and Ferrocene-Filled Single-Walled Carbon Nanotubes. Nanomaterials 2021, 11, 2984, 10.3390/nano11112984.
- Marianna V. Kharlamova; Christian Kramberger; Applications of Filled Single-Walled Carbon Nanotubes: Progress, Challenges, and Perspectives. Nanomaterials 2021, 11, 2863, 10.3390/nano11112863.
