Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Da Sun and Version 5 by Da Sun.
Bacterial ghosts (BGs) are empty bacterial envelopes of Gram-negative bacteria produced by controlled expressions of cloned gene E, forming a lysis tunnel structure within the envelope of the living bacteria.菌影 (BG) 是由克隆基因 E 的受控表达产生的革兰氏阴性菌的空细菌包膜,在活细菌的包膜内形成裂解隧道结构。在全球范围内,BG 已被用作疫苗递送系统和疫苗佐剂。人们对开发基于 BG 用于生物医学应用的新型递送系统越来越感兴趣。
- bacterial ghosts
- gene E
- vaccines
- immune
1. Introduction一、介绍
Delivery systems for drugs, nucleic acids, and other biomolecules are recent developments in biotechnology [1][2]. There is a growing interest in the design and development of novel targeted delivery systems. Bacterial derivatives, including bacterial ghosts (BGs), extracellular vesicles, and alimental toxins, are popular biological nanomaterials that are potential vaccine and drug carriers [3]. These delivery platforms retain many of the advantages of bacteria, including the ability to colonize and target human tissues, enhance immunogenicity of vaccines, and have good loading capacities. Advances in genetic engineering and chemical biotechnology have facilitated the development of different types of BGs, which will be important in immobilized enzyme technology, agriculture and medicine [4]. Recently, BGs have received increased attention as potential candidates for targeted delivery of biomolecules [5][6].药物、核酸和其他生物分子的递送系统是生物技术的最新发展 [ 1 , 2 ]。人们对新型靶向递送系统的设计和开发越来越感兴趣。细菌衍生物,包括细菌幽灵 (BG)、细胞外囊泡和食物毒素,是流行的生物纳米材料,是潜在的疫苗和药物载体 [ 3]]。这些递送平台保留了细菌的许多优点,包括定植和靶向人体组织的能力、增强疫苗的免疫原性以及具有良好的装载能力。基因工程和化学生物技术的进步促进了不同类型 BG 的发展,这将在固定化酶技术、农业和医学中发挥重要作用 [ 4 ]。最近,BG 作为靶向递送生物分子的潜在候选者受到越来越多的关注 [ 5 , 6 ]。
Essentially, BGs are bacterial shells with pores. Genetic engineering or chemical methods can be used to induce the release of cellular contents; hence, they have no nucleic acids, ribosomes, or other components [7]. As such, the structural integrity of surface antigens on most BGs remains intact [8]. The preparation of inactivated vaccines using methods such as formaldehyde and heat treatments can destroy the surface structures of the bacteria [9][10]. Contrarily, BGs prepared by genetic engineering retain all the structural antigens expressed by pathogenic bacteria, which induces very strong, effective humoral and cellular immune responses [11]. The structure of BGs is shown in Figure 1. They contain pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), lipoprotein (LPP), peptidoglycan (PGN), and fimbriae, among others, which are highly conserved structures on the outer cell bacterial wall [12]. Once in the host, BGs are recognized by pattern recognition receptors (PRR) on immune cells, stimulating the production of several immune mediators that induce the maturation of antigen-presenting cells (APCs), such as dendritic cells (DCs) [13][14]. In general, pathogenic bacterial ghost serotypes are well preserved, and high concentrations of BGs can provide a high immunogenicity. Moreover, they can only be purified by washing, centrifugation and freeze-drying [15]. Based on these novel biological characteristics, BGs are potential vaccine delivery systems [16]. They have been progressively adopted in the delivery of nucleic acids, proteins, and chemical drugs [17].本质上,BG 是带有孔的细菌外壳。基因工程或化学方法可用于诱导细胞内容物的释放;因此,它们没有核酸、核糖体或其他成分 [ 7 ]。因此,大多数 BG 上表面抗原的结构完整性保持完整 [ 8 ]。使用甲醛和热处理等方法制备灭活疫苗会破坏细菌的表面结构 [ 9 , 10 ]。相反,通过基因工程制备的 BGs 保留了病原菌表达的所有结构抗原,可诱导非常强、有效的体液和细胞免疫反应 [ 11 ]。BGs的结构如图1所示. 它们包含病原体相关分子模式 (PAMP),例如脂多糖 (LPS)、脂蛋白 (LPP)、肽聚糖 (PGN) 和菌毛等,它们是细菌外壁高度保守的结构 [ 12 ]。一旦进入宿主,BG 就会被免疫细胞上的模式识别受体 (PRR) 识别,刺激多种免疫介质的产生,从而诱导抗原呈递细胞 (APC) 的成熟,例如树突细胞 (DC) [ 13 , 14] ]。一般而言,致病菌鬼血清型保存良好,高浓度的BG可提供较高的免疫原性。此外,它们只能通过洗涤、离心和冷冻干燥来纯化 [ 15]。基于这些新的生物学特性,BG 是潜在的疫苗递送系统 [ 16 ]。它们已逐渐被用于递送核酸、蛋白质和化学药物 [ 17 ]。
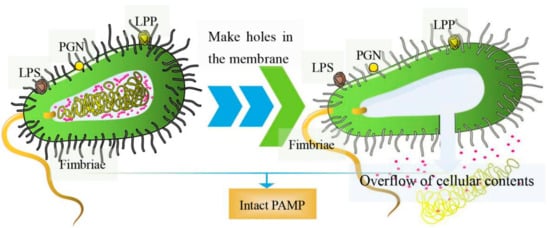
Figure 1. The diagram of BGs. (left) Pores are formed on the surface of bacteria; (right) overflow of cellular contents including nucleic acids, ribosomes, and other components.
图 1. BG 示意图。(左)细菌表面形成孔隙;(右)细胞内容物的溢出,包括核酸、核糖体和其他成分。
2. Methods for BGs Preparation2. BGs 制备方法
2.1. Genetic Engineering2.1. 基因工程
When ΦX174, a single-stranded DNA phage, infects Gram-negative bacteria, it inhibits the activities of the enzyme phospho-MurNAc-pentapeptide translocase (MraY) on the bacteria membrane. This is mediated by lysis protein E, a hydrophobic protein that inhibits the synthesis of PGN in bacterial cell walls [18]. First, the hydrophobic N-terminal binds the inner membrane of the bacterial cell wall. Then, the conformation of protein E changes, binding its hydrophobic C-terminal across the inner and periplasmic spaces to the outer membrane of the cell wall [19]. This disruption causes secondary effects, such as activation of phosphatase activities, and increased membrane mobilities. This increases internal osmotic pressures, inducing the release of cell contents [20]. Currently, the most commonly used method for the preparation of BGs involves cloning lysis gene E (276 bp, accession number: MF426914.1) into expression regulation systems of Gram-negative bacteria, which utilizes the switch function of the control element to control expression [21]. The preparation process for BGs is shown in Figure 2.ΦX174 是一种单链 DNA 噬菌体,当感染革兰氏阴性菌时,它会抑制细菌膜上的磷酸-MurNAc-五肽转位酶 (MraY) 的活性。这是由裂解蛋白 E 介导的,这是一种疏水蛋白,可抑制细菌细胞壁中 PGN 的合成 [ 19 ]。首先,疏水性 N 端结合细菌细胞壁的内膜。然后,蛋白质 E 的构象发生变化,将其疏水性 C 末端穿过内部和周质空间与细胞壁的外膜结合 [ 20 ]。这种破坏会导致二次效应,例如磷酸酶活性的激活和膜迁移率的增加。这会增加内部渗透压,诱导细胞内容物的释放 [ 21]。目前,制备BG最常用的方法是将裂解基因E(276 bp,登录号:MF426914.1)克隆到革兰氏阴性菌的表达调控系统中,利用控制元件的开关功能来控制表达[ 22 ]。BGs的制备过程如图2所示。
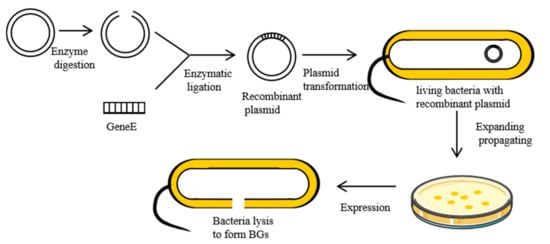

Figure 2. Preparation of the BGs using genetic engineering.图 2.使用基因工程制备 BG。
Constructed BGs are about 1–2 μm long and 0.5–2 μm wide. Lysis protein E connects the inner and outer membranes, minimizing the loss of enzymes in periplasmic spaces. During bacterial lysis, inner and outer membrane structures remain intact. Diameters of transmembrane pores are about 40–200 nm, but, under osmotic pressure, they can reach 500 nm [22]. Pore sizes are determined by sieve sizes of the PGN layer in the cell wall [23].构建的 BG 长约 1-2 μm,宽约 0.5-2 μm。裂解蛋白 E 连接内膜和外膜,最大限度地减少周质空间中酶的损失。在细菌裂解过程中,内外膜结构保持完整。跨膜孔的直径约为 40-200 nm,但在渗透压下,它们可以达到 500 nm [ 23 ]。孔径由细胞壁中 PGN 层的筛孔尺寸决定 [ 24 ]。
跨膜隧道是在细菌分裂位点或细菌极帽处形成的,但主要在细菌位点,这与细菌分裂过程中的Z环形成机制有关。细菌分裂蛋白 FtsZ 是 Z 环形成所必需的 [ 25 ]。维特,A. 等。发现蛋白质 E 介导的裂解发生在 FtsZ 的 GTPase 活性存在下,这与 Z 环结构无关 [ 26 ]。然而,由 FtsZ 的 GTPase 活性引起的蛋白质 E 构象变化的机制应进一步研究。
Transmembrane tunnels are formed at the bacterial division site or polar cap of bacteria, but mostly at the bacterial site, which is related to Z-ring formation mechanisms during bacterial division. The bacterial division protein FtsZ is necessary for Z-ring formation [24]. Witte, A. et al. found that protein E-mediated lysis occurs in the presence of GTPase activities of FtsZ, which is independent of Z-ring structure [25]. However, the mechanisms involved in conformational changes of protein E, which are caused by GTPase activities of FtsZ, should be further investigated.温度敏感性λ pL/pR-cI857 的表达或化学诱导的表达系统(基于Lac 启动子的各种杂种的高效表达系统)通常用作选择成功转化的标记[ 23 ]。对于λPL / PR-子cI857表达,在适当温度下,细菌培养至对数生长期,将温度升高至42之前℃,以诱导基因E [的表达式27,28,29 ]。对于化学诱导表达系统,将IPTG或阿拉伯糖添加到原始培养基中以获得BGs [ 21 ]。获得的BGs被冻干。冻干的 BGs 可以在室温下储存多年 [ 30]。巴里萨尼·阿森鲍尔等人。在发酵罐中培养大肠杆菌直到 OD600 = 0.9,大约需要 90 分钟。在温度敏感的表达系统下,42°C 的温度会诱导蛋白 E 的表达。表达 120 分钟后,裂解效率达到 99.9% [ 31 ]。兰格曼等人。通过将细菌的混合培养基加热至 42°C 来制备 BG。2 小时后,细菌裂解效率达到 99.99% [ 21]。即使有很好的裂解,残留的病原菌也有潜在的危害。因此,需要二次灭活以实现完全裂解。由于紫外线辐射或甲醛灭活会破坏 BG 表面抗原的结构,常用的方法包括添加 β-丙酮 (BPL) 进行灭活、冷冻干燥并在 -20 °C 下储存 [ 10 , 32 ]。庆大霉素和链霉素也用于灭活未裂解的细菌 [ 33 ]。双基因失活也已用于二次失活。朱等人。通过将葡萄球菌核酸酶 A (SNUC) 基因整合到表达 E 融合基因的裂解质粒 (mE-L-SNA) 中,提高了细菌的裂解效率。大肠杆菌的裂解效率在对数生长期达到99.99995%,从而提高了BGs的安全性[ 34 ]。田等人。构造链球菌鸡白痢通过诱导的裂解后熔断抗微生物肽基因,SMAP29,用裂解基因E.二十四小时鬼,他们没有发现任何活细菌[ 35 ]。耶姆等人。构建大肠杆菌突变株(MC4100) 通过删除 ASD 基因,该基因编码参与二氨基庚二酸 (DAP) 合成的天冬氨酸半醛脱氢酶。突变菌株的生长完全取决于添加到培养基中的 DAP 量。基因 E 被诱导后,将目标细菌/BG 在不含 DAP 的 LB 培养基中培养 12 小时,离心、收集并冷冻干燥以完全灭活大肠杆菌[ 36 ]。化学和基因工程方法可以灭活残留的病原菌。
Expressions of temperature-sensitive λ pL/pR-cI857 or chemically induced expression systems (efficient expression systems of all kinds of hybrids based on Lac promoter) are generally used as markers for selection of successful transformation [22]. For λ pL/pR-cI857 expression, at appropriate temperatures, bacteria are cultured to the logarithmic growth phase, before raising the temperature to 42 °C to induce the expressions of gene E [26][27][28]. For chemically induced expression systems, IPTG or arabinose is added to the original culture medium to obtain BGs [20]. Obtained BGs are lyophilized. Lyophilized BGs can be stored at room temperature for many years [29]. Barisani Asenbauer et al. cultured E. coli in a fermentor until OD600 = 0.9, which took about 90 min. Under temperature-sensitive expression systems, a temperature of 42 °C induces the expressions of protein E. After 120 min of expression, lytic efficiency reached 99.9% [30]. Langemann et al. prepared BGs by heating a mixed culture media of bacteria to 42 °C. After 2 h, bacterial lytic efficiency reached 99.99% [20]. Even with the excellent lysis, residual pathogenic bacteria are potentially harmful. Therefore, secondary inactivation is required to achieve complete lysis. Since UV radiation or formaldehyde inactivation can damage the structures of BG surface antigens, the commonly used method involves the addition of β-acetone (BPL) for inactivation, freeze drying, and storage at −20 °C [10][31]. Gentamicin and streptomycin are also used to inactivate non-lysed bacteria [32]. Double gene inactivation has also been used for secondary inactivation. Zhu et al. enhanced the lytic efficiency of bacteria by incorporating the staphylococcal nuclease A(SNUC) gene into a lysis plasmid (mE-L-SNA) expressing the E fusion gene. The lytic efficiency of E. coli at the logarithmic growth stage reached 99.99995%, thus improving the safety of BGs [33]. Tian et al. constructed Streptococcus pullorum ghosts by fusing the antimicrobial peptide gene, SMAP29, with lysis gene E. Twenty-four hours after induced lysis, they did not find any viable bacteria [34]. Hjelm et al. constructed a mutant strain of E. coli (MC4100) by deleting the ASD gene, which encodes aspartate semialdehyde dehydrogenase involved in diaminopimelic acid (DAP) synthesis. The growth of mutant strains is entirely dependent on the amount of DAP added to the culture medium. After gene E was induced, the target bacteria/BGs were incubated in LB medium without DAP for 12 h, centrifuged, collected, and freeze-dried to completely inactivate E. coli [35]. Chemical and genetic engineering methods can inactivate residual pathogenic bacteria.目前,基因 E 裂解系统的表达调控已成功应用于多种革兰氏阴性菌,鼠伤寒沙门氏菌[ 37 ]、肠炎沙门氏菌[ 8 ]、霍乱弧菌[ 38 ]、Pectobacterium cypripedii [ 39 ]、幽门螺杆菌[ 40 ]、胸膜肺炎放线杆菌[ 41 ]、流感嗜血杆菌[ 42 ]、多杀巴氏杆菌[ 43 ]、布鲁氏菌[ 44 ]] 和嗜水气单胞菌[ 45 ] 等。鉴于细菌种类繁多,这些发现意味着任何合适的基因 E 载体,也许所有革兰氏阴性细菌,都可以产生基于裂解的 BG。然而,BG 的制备方法面临着一些挑战。(I) 质粒并非适用于所有革兰氏阴性菌,应针对每个菌株进行研究和修饰。细菌分裂过程中质粒分布不均导致质粒丢失;因此,需要确定基因E是否可以克隆到细菌基因组中。(II) 在制备BGs的过程中,抗生素抗性基因与裂解基因E一起被引入。抗生素抗性基因可以在环境中横向转移[ 46]]。(III) 有许多裂解抗性突变体,尤其是大肠杆菌。大肠杆菌,需要双基因灭活,如结合基因E和基因SNUC[ 47 ]。
Currently, regulation of the expression of the gene E lysis system has been successfully applied against several gram-negative bacteria strains, Salmonella typhimurium [36], Salmonella enteritidis [8], Vibrio cholerae [37], Pectobacterium cypripedii [38], Helicobacter pylori [39], Actinobacillus-pleuropneumoniae [40], Haemophilus influenzae [41], Pasteurella multocida [42], Brucella [43], and Aeromonas hydrophila [44], among others. Given the wide spectrum of bacteria, these findings imply that any suitable gene E carrier, perhaps all Gram-negative bacteria, can produce lysis-based BGs. However, the preparation methods for BGs are associated with several challenges. (I) Plasmids are not applicable to all Gram-negative bacteria, which should be investigated and modified for each strain. Uneven distributions of plasmids during bacterial division leads to plasmid loss; therefore, there is a need to determine whether gene E can be cloned into the bacterial genome. (II) During the preparation of BGs, the antibiotic resistance gene is introduced alongside lysis gene E. The antibiotic resistance gene can be laterally transferred in the environment [45]. (III) There are many lytic resistant mutants, especially E. coli, which requires double gene inactivation, such as combining gene E and gene SNUC [46].基因 E 以外的基因也可用于制备 BG。例如,Ronchel 等人。通过在这些细菌中克隆异源 GEF 蛋白制备了基于恶臭假单胞菌的 BGs [ 48 ]。BGs 还可以通过将表达来自噬菌体 λ 的裂解基因 S、R 和 Rz 的质粒 pDKL02 克隆到大肠杆菌、醋酸钙不动杆菌和假单胞菌[ 49 ] 中来制备。同时,他们将基因E介导的裂解系统与该基因裂解系统进行了比较。他们发现基因E介导的裂解系统产生的核酸可以在体外1小时后完全降解,更有效地制备佐剂。
2.2. 化学方法
2.2. The Chemical Method
“海绵状”方法是制备 BG 的常见化学过程。在这种方法中,使用化学试剂通过细菌细胞壁形成孔。然后,通过离心去除细胞内容物(图 3A)。阿马拉等人。使用低于最低抑菌浓度 (MIC) 的化学品,如 NaOH、SDS、H 2 O 2和 CaCO 3来制备 BG。然后,他们使用 Plackett-Burman 实验设计来优化“海绵状”大肠杆菌幽灵的制备条件[ 50 ]。舍维塔等人。通过在 NaOH、Na 2 CO 3的混合物中培养鲍曼不动杆菌 Ali190制备“海绵”BG和 H 2 O 2 [ 11 ]的溶液。在这些方法下,细胞壁完整性保持完整。萨梅等人。设计了一种新的化学方法,通过在补充有 7% 吐温 80 的培养基中培养沙门氏菌24 小时来制备 BGs [ 51 ]。使用乳酸将培养基的 pH 值降至 3.6。吐温 80 使细菌外膜中的疏水成分溶解,从而形成薄弱区域。这些区域促进了由 pH 值突然降低引起的穿孔形成。在另一种生化方法中,BGs 是通过在溶解在 Na 2 HPO 4溶液中的人工合成模型两亲肽 (MAP) 中培养细菌来开发的。图 3 B) [ 52 , 53 ]。化学过程可以在细菌生长的任何阶段进行,只需稀释以控制 OD 600 = 0.1。基因E介导的裂解效率在对数生长期最好,稳定期裂解导致活细菌存活[ 54 ]。此外,化学方法不限于革兰氏阴性菌,因为它对革兰氏阳性菌和酵母菌也有效[ 55 ]。
The “sponge-like” method is a common chemical process for preparing BGs. In this method, pores are made through bacterial cell walls using chemical reagents. Then, cellular contents are removed by centrifugation (Figure 3A). Amara et al. used less than the minimum inhibitory concentration (MIC) of chemicals such as NaOH, SDS, H2O2, and CaCO3 to prepare BGs. Then, they used the Plackett–Burman experimental design to optimize the preparation conditions for “sponge-like” E. coli ghosts [47]. Sheweita et al. prepared “sponge” BGs by incubating Acinetobacter baumannii Ali190 in a mixture of NaOH, Na2CO3 and a solution of H2O2 [11]. Under these methods, cell wall integrity remains intact. Sameh et al. designed a novel chemical method of preparing BGs by culturing Salmonella for 24 h in a culture media supplemented with 7% Tween 80 [48]. The pH of the medium was reduced to 3.6 using lactic acid. Tween 80 causes the dissolution of hydrophobic components in the outer membrane of the bacteria, thus forming weak areas. These areas facilitate puncture formation caused by the sudden decrease in pH. In another biochemical method, BGs were developed by incubating bacteria in an artificial synthetic model amphiphilic peptide (MAP) dissolved in Na2HPO4 solution (Figure 3B) [49][50]. The chemical process can be performed at any stage of bacterial growth and only requires dilution to control OD600 = 0.1. The efficiency of gene E-mediated lysis was best in the logarithmic growth phase, and lysis in the stationary phase resulted in the survival of live bacteria [51]. Furthermore, the chemical method is not limited to Gram-negative bacteria as it is also effective for Gram-positive bacteria and yeasts [52].
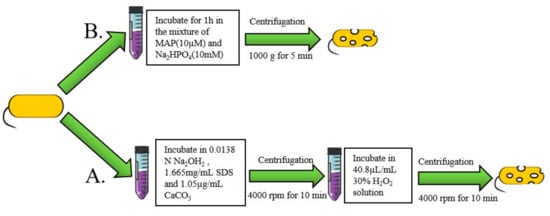

Figure 3. Chemical method for preparation of BGs. (A) Preparation of BGs using the model amphiphilic peptide (MAP). (B) The two-step method for preparation of BGs.图 3. BGs 的化学制备方法。( A ) 使用模型两亲性肽 (MAP) 制备 BG。( B ) 制备 BG 的两步法。
Rapid preparation of BGs using chemical agents eliminates the limitations associated with the use of lysis gene E, particularly genetic restriction-modification [2]. The chemical method is simple, rapid, and does not change the three-dimensional morphology of cells, except for producing holes. However, chemicals may denature surface immunogenic antigens. Excess holes may disrupt the controlled release property. Follow-up experiments should be performed to solve these challenges. Therefore, genetic engineering methods are still the most widely used techniques.
使用化学试剂快速制备 BG 消除了与使用裂解基因 E 相关的限制,特别是基因限制修饰 [ 2 ]。化学方法简单、快速,除产生孔洞外,不改变细胞三维形态。然而,化学品可能会使表面免疫原性抗原变性。过多的孔可能会破坏控释特性。应进行后续实验以解决这些挑战。因此,基因工程方法仍然是应用最广泛的技术。
3. 基于 BG 的疫苗
3. BGs-Based Vaccine
3.1. DNA Vaccines3.1. DNA疫苗
Naked DNA vaccines are limited by their weak immunogenicity. To elicit stronger immune response, they are usually combined with various adjuvants, such as alum. However, the currently used adjuvants have various limitations and the adjuvant options available to any recombinant vaccine manufacturer are very limited. However, carrier transport can enhance its immunogenicity and efficiency [53]. Currently, nucleic acid vectors, including viruses (influenzavirus, adenovirus, poliovirus, etc), fungi (Saccharomyces), and bacteria (Bacillus Calmette Guerin), among others, are commonly used. As carriers, BGs can be internalized by various cells, such as mouse macrophage raw 264.7, HCDECs, DCs, and Caco-2 [54][55][56]. In vitro cell experiments have shown that 60% of macrophages (raw 264.7) can internalize BGs containing reporter plasmids and then express green fluorescent proteins [57]. DCs can also internalize BGs and secrete IL-12 to activate Th1 immune responses [58]. The internalization and activation of BGs by APC cells provides a new strategy for vaccination and in situ immunotherapy. BGs carrying plasmids adopt the diffusion strategy (plasmid DNA diffuses through the lytic pore into the BGs) [57], and the nucleic acid can nonspecifically bind with BGs (The negatively charged DNA binds to positively charged groups in the inner membrane, such as amines). Each BG can load 4000–5000 copies of plasmid DNA (from medium to large plasmids) [59]. These in vitro experiments have proven that BGs are feasible for nucleic acid delivery. In mice models, there have been many studies of BG-based nucleic acid vaccines. Zhou et al. used BGs as a delivery system to prepare an effective DNA vaccine for the prevention of C. psittaci infection [60]. This BG-based DNA vaccine induced a stronger humoral immunity (IgG upregulation) and cellular immunity (Th1 type immune related indicator upregulation) than the naked DNA vaccine and BGs. Jiao et al. used the Salmonella ghost to prepare DNA vaccines to prevent Neisseria gonorrhoea [61]. They found that BG-based DNA vaccines induced stronger humoral immunities (IgG upregulation) and lymphocyte proliferation, relative to naked DNA vaccines and BGs alone. In a model experiment with bone marrow-derived DCs (BMDCs), Jiao et al. cultured the vaccine with BMDCs [62]. They found that the BG-based DNA vaccine promoted greater DCs maturation and activation (up regulation of cell surface costimulatory molecules CD80, CD86, CD40, and MHC-II) than the naked DNA vaccine and BGs alone. These results show that DNA vaccines prepared with BG-loaded plasmids have a better stimulatory effect on both humoral and cellular immunities, relative to naked DNA vaccines. Cao et al. used DH5α ghosts loaded with plasmids containing five exogenous fragments (including invariant chain-like protein [Iclp] gene) to prepare double-targeted DNA vaccines for oral immunization of grass carp [63]. Plasmids with endogenous Iclp easily enter the MHC-II antigen presentation pathway. Immunization with the double-targeted DNA vaccine substantially increases the activities of three innate immune parameters (SOD, LZM and C3) in serum and intestinal mucus. Moreover, the relative survival rate of the experimental group reached 81.11%, demonstrating the efficiency of the vaccine against Vibrio strains. These studies show that antigenic genes in BGs can be internalized and expressed by APCs, and foreign genes can be derived from multiple plasmids or a plasmid containing the fusion gene fragment. The multigene BGs vaccine induced stronger immune responses than the naked DNA vaccine.裸 DNA 疫苗受到其弱免疫原性的限制。为了引起更强的免疫反应,它们通常与各种佐剂,如明矾结合。然而,目前使用的佐剂有各种限制,任何重组疫苗制造商可用的佐剂选择都非常有限。然而,载体转运可以增强其免疫原性和效率[ 90 ]。目前常用的核酸载体包括病毒(流感病毒、腺病毒、脊髓灰质炎病毒等)、真菌(酵母菌)、细菌(卡介苗)等。作为载体,植物园可通过各种细胞,例如小鼠巨噬细胞内化的RAW 264.7,HCDECs,DCS和的Caco-2 [ 76,86,91 ]。体外细胞实验表明,60% 的巨噬细胞(原始 264.7)可以内化含有报告质粒的 BG,然后表达绿色荧光蛋白 [ 92 ]。DC 还可以内化 BG 并分泌 IL-12 以激活 Th1 免疫反应 [ 93 ]。APC 细胞对 BG 的内化和激活为疫苗接种和原位免疫治疗提供了一种新策略。携带质粒的BGs采用扩散策略(质粒DNA通过裂解孔扩散到BGs中)[ 92 ],核酸可以与BGs非特异性结合(带负电荷的DNA与内膜中带正电荷的基团结合,如胺类) )。每个 BG 可以加载 4000–5000 个质粒 DNA 拷贝(从中型到大型质粒)[ 94]。这些体外实验已经证明BGs对于核酸递送是可行的。在小鼠模型中,已经有很多基于BG的核酸疫苗的研究。周等人。使用 BG 作为传递系统来制备有效的 DNA 疫苗以预防鹦鹉热感染 [ 79 ]。这种基于 BG 的 DNA 疫苗比裸 DNA 疫苗和 BG 诱导更强的体液免疫(IgG 上调)和细胞免疫(Th1 型免疫相关指标上调)。焦等人。所使用的小号almonella鬼以制备DNA疫苗以预防淋病奈瑟菌[ 80]。他们发现,相对于裸 DNA 疫苗和单独的 BG,基于 BG 的 DNA 疫苗诱导更强的体液免疫(IgG 上调)和淋巴细胞增殖。在骨髓衍生 DCs (BMDCs) 的模型实验中,Jiao 等人。用 BMDCs 培养疫苗 [ 95 ]。他们发现基于 BG 的 DNA 疫苗比裸 DNA 疫苗和单独的 BG 促进了更大的 DC 成熟和激活(细胞表面共刺激分子 CD80、CD86、CD40 和 MHC-II 的上调)。这些结果表明,与裸 DNA 疫苗相比,用负载 BG 的质粒制备的 DNA 疫苗对体液和细胞免疫具有更好的刺激作用。曹等人。使用过的DH5α装载含有五个外源片段(包括不变链状蛋白[Iclp]基因)的质粒的ghosts,制备用于草鱼口服免疫的双靶向DNA疫苗[ 81 ]。带有内源性 Iclp 的质粒很容易进入 MHC-II 抗原呈递途径。双靶向 DNA 疫苗的免疫显着增加了血清和肠粘液中三种先天免疫参数(SOD、LZM 和 C3)的活性。而且,实验组的相对存活率达到了81.11%,证明了疫苗对弧菌的有效性。菌株。这些研究表明,BGs 中的抗原基因可以被 APCs 内化和表达,外源基因可以来自多个质粒或含有融合基因片段的质粒。多基因 BGs 疫苗比裸 DNA 疫苗诱导更强的免疫反应。
3.2. Protein Antigen Vaccines3.2. 蛋白抗原疫苗
BGs serve as carriers for protein vaccines in two ways. (I) Multi epitope peptide BGs: through genetic engineering, the antigen and protein target is displayed on the surface to improve its immunogenicity and targeting. (II) Non-recombinant BGs mixture: when the antigen is co-incubated with BGs, the protein non-specifically binds the intima. However, to obtain strong immune responses, optimal doses of proteins and BGs should be investigated further. Tuntufye HN et al. found that BG-based recombinant vaccines of ferri-siderophore receptors protected chickens against avian pathogenic E. coli APEC infection [64]. They found that both the recombinant BGs vaccine and the non-recombinant mixture vaccine could significantly increase IgG immune responses and reduce mortality after infection. Moreover, the mortality rate for recombinant BGs chickens was lower, relative to that of the non-recombinant mixture vaccine. Sai Gong et al. developed a vaccine against hand-foot-and-mouth disease by expressing antigenic proteins of Entero virus 71 and Coxsackie virus in the outer membrane protein A(OMPA) of E. coli O157: H7 [15]. The vaccine increased IgG and IgA secretion, thereby inducing mucosal immunity. Moreover, the vaccine candidate protected mice against E. coli infection. In addition to expressing protein antigens on the outer membrane of BGs, Riedmann et al. enhanced cellular immune responses via intestinal or lung inoculation of BGs developed by fusing the Haemophilus influenzae (NTHi) antigen (OMP26) into the S layer or periplasmic space of E. coli [41]. In conclusion, endomembrane proteins, periplasmic space proteins and outer membrane proteins on BGs are antigenic, and they can induce immunogenicity. Compared to the protein subunit vaccine, BGs adjuvants significantly enhance the immunogenicity of antigenic proteins [19].BGs 以两种方式作为蛋白质疫苗的载体。(I)多表位肽BGs:通过基因工程,将抗原和蛋白靶点展示在表面,提高其免疫原性和靶向性。(II) 非重组BGs混合物:当抗原与BGs共孵育时,蛋白质非特异性结合内膜。然而,为了获得强大的免疫反应,应进一步研究蛋白质和 BG 的最佳剂量。Tuntufye HN 等。发现基于 BG 的铁-铁载体受体重组疫苗可以保护鸡免受禽类致病性大肠杆菌APEC 感染 [ 83]]。他们发现重组BGs疫苗和非重组混合疫苗均可显着提高IgG免疫反应并降低感染后死亡率。此外,与非重组混合疫苗相比,重组BGs 鸡的死亡率较低。赛工等。通过在大肠杆菌O157: H7 的外膜蛋白 A (OMPA) 中表达肠病毒71和柯萨奇病毒的抗原蛋白,开发了一种针对手足口病的疫苗[ 15 ]。该疫苗增加了 IgG 和 IgA 的分泌,从而诱导了粘膜免疫。此外,候选疫苗可以保护小鼠免受大肠杆菌的侵害感染。除了在 BGs 的外膜上表达蛋白质抗原外,Riedmann 等人。通过将流感嗜血杆菌(NTHi) 抗原 (OMP26) 融合到大肠杆菌的 S 层或周质空间中,通过肠道或肺部接种 BG 增强细胞免疫反应[ 42 ]。总之,BGs上的内膜蛋白、周质间隙蛋白和外膜蛋白具有抗原性,可以诱导免疫原性。与蛋白质亚单位疫苗相比,BGs 佐剂显着增强了抗原蛋白质的免疫原性 [ 20 ]。
Currently, there is increasing attention on how to improve the protein loading capacities of BGs (Figure 5). To achieve this, attempts have been made to express streptavidin in the inner membrane of BGs, whereas the target protein is phthalated with biotin. The specific interaction between biotin and streptavidin immobilizes the target protein in the inner membrane. In other studies, the protein was attached to the bacterial membrane through genetic engineering. For example, Sührer et al. attached galactosidase anchors on BGs containing cytochrome b5 to immobilize the enzyme [65]. In other studies, BGs have been sealed by fusions with membrane vesicles [66].目前,如何提高BGs的蛋白质负载能力越来越受到关注(图4)。为了实现这一点,已经尝试在 BG 的内膜中表达链霉亲和素,而目标蛋白是用生物素邻苯二甲酸化的。生物素和链霉亲和素之间的特异性相互作用将靶蛋白固定在内膜中。在其他研究中,蛋白质通过基因工程附着在细菌膜上。例如,Sührer 等人。将半乳糖苷酶锚定在含有细胞色素 b5 的 BG 上以固定酶 [ 96 ]。在其他研究中,BG 已通过与膜囊泡融合来密封 [ 97 ]。
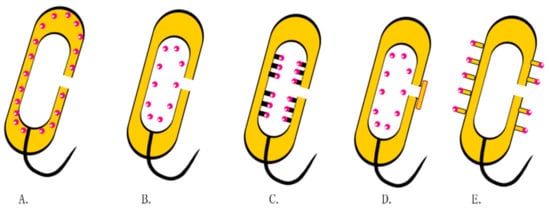

Figure 4. Drug loading strategies in BGs. (A) Expressions of proteins or antigens in the periplasmic space through genetic engineering. (B) Non-specific binding of drugs to the inner membrane. (C) Attachment of exogenous proteins or antigens onto the inner membrane using streptavidin. (D) BGs encapsulated with membranous vesicles. (E) Multiepitope peptide BGs.图 4. BG 中的载药策略。( A ) 通过基因工程在周质空间表达蛋白质或抗原。( B ) 药物与内膜的非特异性结合。( C ) 使用链霉亲和素将外源蛋白质或抗原附着在内膜上。( D ) BGs 被膜囊泡包裹。( E ) 多表位肽 BG。
BGs can either be mixed with the antigen of interest, or the antigen can be expressed in a Gram-negative bacterium to be turned into BGs, thereby creating a bacterial shell with integrated antigens. Given the success of nucleic acid vaccines for COVID-19, BG-based vaccine research should fully utilize the natural intrinsic adjuvant effect of BGs in its vaccine candidates.BGs 可以与感兴趣的抗原混合,或者抗原可以在革兰氏阴性细菌中表达以转化为 BGs,从而创建具有整合抗原的细菌外壳。鉴于 COVID-19 核酸疫苗的成功,基于 BG 的疫苗研究应充分利用 BG 在其候选疫苗中的天然内在佐剂作用。
