Chitosan is one of the most well-known and characterized materials applied in tissue engineering. Due to its unique chemical, biological and physical properties chitosan is frequently used as the main component in a variety of biomaterials such as membranes, scaffolds, drug carriers, hydrogels and, lastly, as a component of bio-ink dedicated to medical applications. Chitosan’s chemical structure and presence of active chemical groups allow for modification for tailoring material to meet specific requirements according to intended use such as adequate endurance, mechanical properties or biodegradability time.
- chitosan
- biopolymer
- biomedicine
1. Introduction
Chitosan as a biopolymer has several useful physicochemical properties such as solubility, reactivity, adsorption, and crystallinity. In turn, its biological properties include biodegradability, antimicrobial activity, cytocompatibility, lack of toxicity, and fungicidal effects. Furthermore, the advantage of showing anti-cholestemic activity, antioxidant activity, macrophage activation, anti-inflammatory activity, angiogenesis stimulation, muco-adhesion, antitumor, granulation, scar formulation, hemostatic action and wound healing stimulation makes it a tremendously good candidate for numerous applications in biomedicine [1][2][3][4][4,5,6,7]. The role of chitosan in the pharmaceutical industry has been extensively explored. The exemplary use of chitosan in the field of medicine includes cartilage repair, bone tissue engineering, liver tissue engineering, vascular tissue engineering, drug delivery, wound healing, regenerative medicine, gene therapy, biosensing, excipient for tablets, controlled release dosage, absorption enhancer, developing micro/nanoparticles, dental implants, dental plaques, and dentifrices. Other potential possibilities of CS (chitosan) usage are in food, chemicals, cosmetics, water treatment, metal extraction and recovery, biochemical, agricultural, and environmental uses [5][6][8,9]. Consequently, chitosan has approval for application as a biomaterial by the Food and Drug Administration (FDA) in the USA and regulatory bodies of other countries and is currently used in a number of commercial products from hemostatic bandages to dietary aides [7][10].
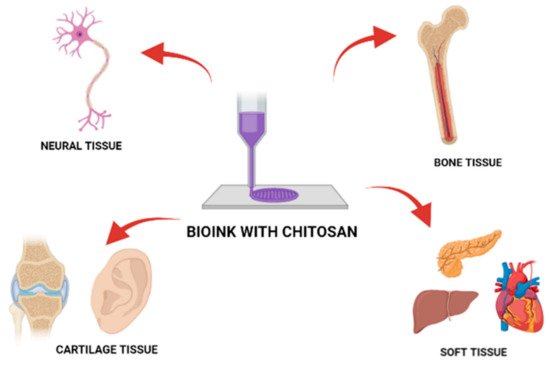
3D printing and bioprinting fields have experienced rapid development in recent years. Chitosan is one of the best 3D printing candidates due to its desirable physicochemical properties and essential features for cell adhesion, Extracellular Matrix (ECM) deposition, and finally tissue regeneration. However, the 3D printing of chitosan-based hydrogels is still under early exploration. The combination of chitosan-based hydrogels and 3D printing holds much promise in the development of next-generation biomedical implants [8][14].
2. Chitosan Blends with Other Materials
The field of biomaterials must meet requirements such as appropriate mechanical parameters, biocompatibility, and stability in the aquatic environment [9][103]. To improve the properties or create new ones, chitosan can be combined with other natural or synthetic polymers. Obtaining blends by mixing polymers allows the production of components with specific, desired properties and applications. The most frequently improved properties in biomaterials by the addition of chitosan are increasing hydrophilicity, mechanical properties, antibacterial properties, and improving blood compatibility [10][106]. Another method of improving the characteristics of chitosan is to subject it to a wide range of modifications.
3. Chitosan Derivatives
The most common modification of the chitosan is acylation. It refers to the reaction of chitosan with a variety of organic acids and its derivatives by adding aliphatic or aromatic acyl groups to the molecular chitosan chain. The acylation reaction destroys the chitosan’s intermolecular and intramolecular hydrogen bonding which weakens its crystallinity and enhances water solubility.
The introduction of carboxyalkyl groups into the structure of chitosan as carboxymethyl is mainly carried out to increase the solubility level of chitosan. The reaction occurs either at the C6 hydroxyl group or at the NH2 moiety obtaining N-CMC, O-CMC, or N-O-CMC as products. CMC is an amphoteric and water-soluble chitosan derivative widely used in biomedicine. CMC as well as chitosan has excellent biocompatibility, biodegradability, and antioxidant activity. Therefore, these amphoteric polymers can be loaded with hydrophobic drugs and display strong bioactivity [11][189].
The quaternary ammonium is a positively charged hydrophilic group. Its addition to chitosan not only increases water solubility but also increases chargeability. Chitosan is positively charged at PH under 6.5, whereas quaternized chitosan is still permanently positively charged at PH above 6.5 The quaternization occurs with C2–NH 2 and consists of introducing alkyl groups in place of the amino groups of chitosan. The most popular quaternization of chitosan products is N-Trimethyl Chitosan (TMC). It is considered to be one of the strongest existing mucoadhesive polymers. This is due to the presence of cationic groups in its chain [12][197]. Quaternary ammonium salt can be used for the quaternization reaction. It weakens hydrogen bonds and increases charging strength, which occurs with increasing water solubility. Quaternary ammonium chitosan salt has better antimicrobial, biocompatible, biodegradable, and non-toxic properties. It can penetrate mucus layers and bind to epithelial surfaces [13][198]. It is worth adding that the higher the DS degree, the better the water solubility properties and the higher the material potential [14][199].
Thiolation is the reaction between primary amino groups of chitosan with coupling reagents that contain thiol groups [15][200]. Primarily, thiolated chitosan has a greater solubility in the aqueous environment but also has improved permeation and displays in situ gelling properties [16][201]. Thiolated chitosan derivatives have enhanced mucosal adhesion properties due to the formation of covalent bonds between free thiol groups and cysteine-containing glycoproteins in mucus [17][202]. The in situ gelling ability makes thiolated chitosan suitable not only for nose-to-brain applications but also for the elaboration of scaffolds. Not only chitosan but also metha-crylamide chitosan can be thiolated. As a result of such a reaction a porous, biodegradable material is created, which has an excellent effect on cell growth and neural stem target differentiation in 3D. The most effective method of making thiolated chitosan derivatives is the annealing method. Such a derivative has stronger adhesion, hydration ability, and drug release than other preparation methods [18][203].
To achieve copolymerization of chitosan, it may be grafted onto it. A sample product of such reaction is polyethylene glycol (PEG)-grafted chitosan derivative which gives a significant increase in solubility over a wide PH range. Moreover, it shows increased muco-adhesion [19][204]. Other polymers that have been grafted to chitosan for Central Nervous System (CNS) application are gelatin, polylactic-co-glycolic acid (PLGA), poly (3,4 ethylene-dioxy-thiophene) (PEDOT), alginate, and agarose.
43. Chitosan as a Component of Biomaterials
54. Chitosan as a Bio-Ink Composition Material
| Chitosan Blends | Material Dedication | Rheology | Toxicity/ Cell Proliferation |
Biodegradability Swelling Ratio |
Comments | Bibliography |
|---|
| Carboxymethyl Chitosan-Based Bioink: Ethylenediaminetetraacetic acid (EDTA) stabilized with 0.5 M calcium chloride |
Cartilage tissue | Storage modulus G′ at 23 °C: 112 kPa | Rabbit chondrocytes; flow cytometry; 95:9 ± 1:3% After 36 h seeded on mesh; similar proliferation rate between the control group (9:9 ± 0:7%) | Swelling ratio: 14–22% weight increase after 22 days in water | Bioprinting of scaffolds for cells | [22] | [277] | ||||
| Cell-Laden Thermosensitive Chitosan Hydrogel Bioink: β-glycerophosphate Potassium phosphate Sodium bicarbonate |
Development of chitosan-based bioink | Storage modulus G′ at 36 °C: around 1000 Pa | Human periodontal ligament stem cells; WST (Colorimetric assay for the nonradioactive quantification of cell proliferation, cell viability, and cytotoxicity )assay showed that there was no significant difference in cell viability until day five | Lack of information | Cell encapsulation is associated with minimal cytotoxicity | [23] | [278] | ||||
| Cell-laden hydrogels, bioink: Potato starch |
3D bioprinting scaffolds for neural cell growth | Neuro-2a, mouse neuroblastoma cells LDH (Lactate Dehydrogenase)assay kit and fluorescent microscopy: viability after 10 days-10% and lower |
Degradation time is decreasing with the addition of the potato starch component | Chitosan dissolution and crosslinking must be optimized | [24] | [279] | |||||
| BMSCs-laden gelatin/sodium alginate/carboxymethyl chitosan hydrogel: Gelatin Sodium alginate Carboxymethyl chitosan |
3D bioink for tissue scaffolds | Young modulus: 80–120 mPa | Bone mesenchymal stem cells (BMSC); Live/Dead cells staining: 85% of the printed cells were viable at 0 and 2 days of culturing | Biodegradation in 60 days in the physiological environment: 35–50% mass loss | Bioink showing antimicrobial properties towards | E.coli | [25] | [280] | |||
| Fabrication of hydroxybutyl chitosan/oxidized chondroitin sulfate hydrogels: Hydroxybutyl chitosan (HBC) Oxidized chondroitin sulfate via Shift base reaction |
Cell delivery system for cartilage tissue engineering | Turn into stabile hydrogel at 35 °C–40 °C, Storage modulus G′: −150–300 Pa for 50 mg/mL HBC concentration | Mesenchymal stem cells, Live/ Dead assay after via fluorescent microscopy; Cell viability was verified inside the hydrogels in 14 days, showing gradually spreading in the hydrogel with the appearance of pseudopodia | Lack of information | Injectable hydrogel with a porous structure of average 100 µm pore size was developed to form a microporous hydrogel | [26] | [281] | ||||
| DLP printing photocurable chitosan: Methacrylic anhydride Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) |
Photocurable bioink for digital light processing (DLP) technology for tissue engineering | Stress-strain for CHIMA (methacrylated derivative of chitosan) 33.6% 80 kPa; CHIMA 44.6% 33.6% −150 kPa | Human umbilical vein endothelial cells (HUVECs); LIVE/DEAD Viability/Cytotoxicity kit: Viability for 4 examined samples oscillated around 90% after 3 days from incubation | The swelling ratios of hydrogels 11.7–33.6% DS exhibit a decreased trend from 500% to 150% 2 during the incubation time |
The CHI-MA (1 wt%) with 33.6% DS was selected as the photocuring bioink for DLP | [27] | [282] | ||||
| Photocurable chitosan as bioink Methacrylated chitosan Β-glycerol phosphate salt (β-GP) |
Bioink for cellularized therapies towards personalized scaffold architecture |
40 s of exposition at 37 °C initiate crosslinking bioink: Storage modulus G’: −90–100 Pa | NIH, 3T3, Saos-2, SH-SY5Y cell lines; LIVE/DEAD Viability/Cytotoxicity kit, fluorescence microscopy: viability: around 95–115% compering to control, after 24 h | Decreased mass of 55% after 14 days of incubation in the cultured medium at 37 °C; thermogravimetric analysis | Bioink did not adversely affect the hosting cells and allowed cell proliferation and organization towards tissue formation. |
[28] | [196] | ||||
| Chitosan ducts fabricated by extrusion-based 3D printing Formic acid Acetic acid Glycolic acid Lactic acid |
Soft tissue restoration | Young modulus: 12.38 ± 1.19 MPa |
MTT test on L929 mouse fibroblast cell line for 24 h cell viability of CS ducts prepared by 30% GA close to 90% | Stable after soaking in two weeks in Tris-HCl with the addition of lysozyme | The 30 wt.% GA was optimal based on tensile properties and preliminary cytotoxicity | [29] | [283] | ||||
| Chitosan-calcium phosphate inks: Calcium Phosphate Acetic acid orthophosphate solutions |
Bioinks as potential bone substitute | For all other inks, Loss modulus G″ were higher than G′ from the start, thus the inks were liquid-like |
Not tested | Lack of information | More printable inks are obtained with higher chitosan concentration (0.19 mol·L | −1 | ). | [30] | [284] | ||
| Cell-Laden Nanocellulose/Chitosan-Based Bioinks: Glycerophosphate Hydroxyethyl cellulose Cellulose nanocrystals |
Bioprinting and enhancing cell differentiation for bone tissue | Viscosity in the range of 30 Pa·s–6 × 10 | 4 | Pa·s; Yield stress 412.35 ± 45.35 pa | MC3T3, a pre-osteoblast cell line; Live/Dead cell staining kit; after 7 days incubation in media at 37 °C there is neither significant proliferation nor cell toxicity | The shrinkage of scaffolds after 24 h incubation in DMEM (Dulbecco’s Modified Eagle Medium) at 37 °C ranges between 30–34% | [31] | [285] | |||
| Natural based poly(gamma-glutamic acid)/Chitosan bioink: Poly(gamma-glutamic acid) |
Alternative to other materials used in 3D bioprinting | Storage modulus G′ around 50 Pa and 30 Pa for 4.5% and 6% Chitosan hydrogels | Human adult fibroblast: Cell viability after 14 days incubation of DMEM on bioink around 80% | 35% Mass loss after 35 days incubation in cell medium | FTIR analysis demonstrated Gamma-PGA/Cs interpolyelectrolyte complex formation |
[32] | [286] | ||||
| A writable bioink under serum culture media: Catechol Vanadyl ions |
Polymer for 3D printing | Storge modulus G′ value of the V-Chi-C gels at 1 Hz was gradually enhanced up to 6 ± 0.5 × 10 | 6 | Pa at 168 hrs from 69 ± 18 Pa at 0 hr | LIVE⁄DEAD | ® | Viability/Cytotoxicity Kit; 90% L929 cells viability after 5 days incubation on scaffolds | Weight loss down to 50% of initial mass at 12 h of incubation in PBS, and remained constant (40%) for next 7 days | [26] | [281] |
