You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Lubos Danisovic.
Mesenchymal stem cells/stromal cells (MSCs), which have been applied in implantology and periodontology. MSCs, with their multilineage differentiation potential (differentiation into osteocytes, chondrocytes, adipocytes, muscle cells, and even neurocytes) are widely available from various tissues sources.
- alveolar bone regeneration
- tissue engineering
- pluripotent stem cells
- embryonal stem cells
1. Introduction
The restoration of severe periodontal defects, such as damage to the alveolar bone or soft periodontal tissue, is still a complex and challenging field for clinicians. There are various causes of bone defects including congenital anomalies, medications, local inflammation, periodontitis, traumatic injuries, malignancies, and dental surgical interventions. The traditional therapy to overcome bone atrophy relies on the use of more or less invasive techniques. Autologous alveolar bone grafts represent “the gold standard” due to their osteogenic, osteoinductive, and osteoconductive properties. Therefore, they are the first-choice option in the reconstruction of large bone defects [1]. However, the use of autografts is usually associated with donor site morbidity, graft failure, and immunological rejection. A limited source of graft tissue is also a problem that needs to be addressed. Moreover, this procedure is painful and often results in prolonged hospitalization. There are some other bone tissue substitutes, such as allografts and xenografts; however, their disadvantages include the possibility of immune rejection and pathogen transmission from the donor to the host. The application of synthetic grafts is also limited because of their non-optimal integration with native tissue, which can often lead to graft failure [2,3,4][2][3][4]. As of today, an ideal technique with the ability to completely regenerate harmed bone tissue has not been found.
A promising alternative in reconstructing alveolar bone tissue is tissue engineering techniques and stem cell-based regenerative therapies, where the key factor is the most suitable combination of cells, scaffolds, and signaling molecules [5] (Figure 1).
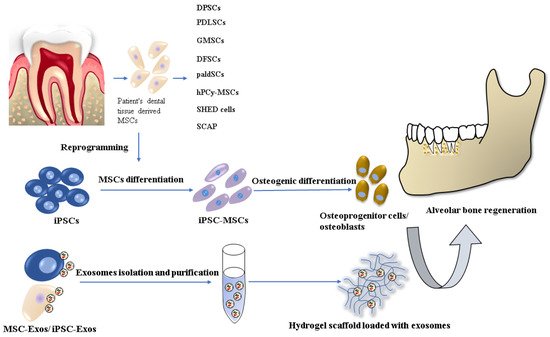
Figure 1.
Stem cell-based tissue engineering methods for alveolar bone regeneration.
There are already several dentistry regenerative approaches based on the most commonly used stem cell type: mesenchymal stem cells/stromal cells (MSCs), which have been applied in implantology and periodontology. MSCs, with their multilineage differentiation potential (differentiation into osteocytes, chondrocytes, adipocytes, muscle cells, and even neurocytes) are widely available from various tissues sources. The MSCs used in oral and maxillofacial regions are usually harvested from bone marrow, adipose tissue, and dental tissue [6,7,8][6][7][8]. However, their cultivation and expansion are time-consuming, resulting in a senescent cell population. Other cell lineages, which has been used in in vitro and in vivo animal studies are embryonal stem cells (ESCs) and induced pluripotent stem cells (iPSCs). Nevertheless, the use of ESCs is related to serious ethical concerns involving the controversial in vitro human blastocyst destruction. On the other hand, iPSC research promises great potential for dental tissue regeneration thanks to its similar characteristics to ESCs but with no ethical issues. Precisely, iPSCs can be generated from several adult somatic cells specific for the concrete patient. Despite the many advantages of iPSC technology, there are still several safety challenges, such as teratoma formation or malignant transformation, which have to be solved before their clinical application [9,10][9][10].
2. Osteogenic Potential of Dental Tissue-Derived MSCs
The regenerative process of alveolar bone reconstruction is based on ossification during embryonal development, where the osteoblasts differentiated from MSCs produce osteoid, an unmineralized bone matrix, with bone mineralization following. Moreover, MSCs with their paracrine secretion of cytokines and growth factors are also able to enhance bone regeneration indirectly. Released factors, such as tumor necrosis factor-α (TNF-α), platelet-derived growth factor (PDGF), interleukin-1 (IL-1), and IL-6, can initiate further activation of MSCs and their recruitment in regenerated sites [11,12][11][12]. Therefore, successful bone tissue engineering should involve a combination of abundant MSCs/ osteoprogenitor cells, a suitable mixture of biofactors to induce osteogenic differentiation, and scaffolds based on biomaterials. For dental tissue regeneration, the most eligible are MSCs derived from various dental sources, such as dental pulp—dental pulp stem cells (DPSCs) [13]; periodontal ligament—periodontal ligament stem cells (PDLSCs) [14,15][14][15]; gingiva—gingival mesenchymal stem cells (GMSCs) [16,17][16][17]; dental follicle or bilayered Hertwig’s epithelial root sheath—dental follicle stem cells (DFSCs) [18]; subepithelial palatal soft tissue—palatal-derived stem cells (paldSCs) [19,20][19][20]; periapical cyst tissue—human periapical cyst mesenchymal stem cells (hPCy-MSCs) [21,22][21][22]; and stem cells from exfoliated deciduous teeth (SHED cells) and from human root apical papilla (SCAP) [23] (Table 1).
Dental-derived MSCs display the same characteristics as bone marrow-derived MSCs (BM-MSCs); furthermore, they possess immunomodulatory and anti-inflammatory advantages in the local dental tissue environment [24].
The regenerative activity of MSCs following transplantation can be performed in several ways. It can be either by the direct engraftment and differentiation of MSCs into newly formed tissue or by immunomodulatory regulation of direct or indirect secretory signaling [25]. Bajestan et al. (2017) investigated the effectiveness of stem cell therapy for reconstructing alveolar cleft and trauma defects in adults. In a randomized controlled clinical trial, eighteen patients whose therapy was based on conventional autogenous block grafts or transplantation of autologous BM-MSCs processed by ixmyelocel-t were involved. Four months after transplantation, the grafting sites were re-entered to evaluate the implant stability, and in six months, the successfulness of the regenerative process was analyzed. In general, the results showed the capability of stem cell therapy to safely induce bone regeneration, nevertheless, with limited capacity in case of large alveolar defects [26].
DPSCs as a subpopulation of MSCs give rise to odontoblasts during tooth development. These cell populations have been the most studied dental stem cells for bone regeneration. The majority of studies reported the osteoinductive potential of DPSCs in vitro and in vivo [7,27,28][7][27][28]. Moreover, d’Aquino et al. (2007) found out for the first time that 30% of osteoblasts differentiated from DPSCs expressed not only osteocalcin but also specific antigens for endothelial cells, such as flk-1, CD54, von-Willebrand factor, CD31 (PECAM-1), and angiotensin-converting enzyme. Therefore, the authors transplanted DPSCs placed on bone chips into an in vivo model of immunocompromised rats. Interestingly, after 60 days, the formation of vascularized bone tissue was observed similar to adult bone, making them an ideal candidate for bone tissue replacement [29]. Tanikawa et al. (2020) performed a unique transplantation of deciduous DPSCs associated with hydroxyapatite-collagen sponges into the unilateral alveolar bone defect of six patients aged 8–12. After six months, during the post-operative evaluation, bone healing with no ectopic bone formation and graft loss was observed in all patients. However, the success of this first clinical trial using deciduous DPSCs was hindered by a small group of participants [30]. Paduano et al., 2021 demonstrated for the first time that the osteogenic capacity of DPSCs and DFSCs can be increased by their dedifferentiation into stem cell-like state (Dediff-DPSCs) under physiological conditions. Comparing the osteogenic potential between redifferentiated Dediff-DPSCs/DFSCs and osteogenic differentiated DPSCs/DFSCs revealed elevated expressions of Runx-2, osteocalcin, and osteonectin in redifferentiated DPSCs. Moreover, redifferentiated DPSCs/DFSCs exhibited higher formation of calcium nodules. Therefore, this study offers a new dedifferentiation approach to enhance the osteogenic potential of DPSCs/DFSCs without gene manipulation [31].
The osteogenic capacity of paldSCs in alveolar bone regeneration was proved by Grimm et al. (2014) in their clinical study with 30 patients. The authors combined allogeneic bone blocks with human adult paldSCs and implanted them into the alveolar bone defect. According to the performed analyses, the osteoinductive effect of paldSCs was manifested by improvement of vertical alveolar bone augmentation [19].
The DFSCs are tooth germ cells originating from the neural crest, which can be isolated from wisdom teeth. Due to their unique neuroectodermal origin, the DFSCs are direct precursors of periodontal tissues, such as periodontal ligaments, cementum, alveolar bone, as well as salivary gland cells [32]. Most in vitro studies proved the osteogenic properties of DFSCs in an appropriate osteoinductive medium [33,34,35][33][34][35]. However, there are only a few in vivo studies using murine or porcine models. Honda et al. (2011) used pellets with DFSCs to repair critical-sized calvarian defects in immunodeficient rats and observed bone formation similar to intramembranous ossification. The results of the immunohistological analyses showed the formation of a new bone matrix surrounded by osteoblasts [36].
GMSCs, quite novel postnatal stem cells, have attracted more attention during the past few years, thanks to their easy isolation, high proliferation capacity, and stable phenotype. Surprisingly, they can maintain telomerase activity in a prolonged culture with no tumorigenesis. According to the literature, GMSCs have high potential in the regeneration of alveolar bone defects, periodontium, oral neoplasms, and peri-implantitis [37,38,39][37][38][39]. To date, several studies have been published concerning the use of GMSCs in dental tissue regeneration. An interesting study was authored by Sun et al. (2019), who had systematically transplanted human GMSCs into a C57BL/6J mice model of severe periodontitis via the tail vein to observe their possible interaction with periodontal tissue. GFP-stained GMSCs were inserted in the second maxillary molar by a silk thread ligature. Four weeks post-transplantation, the histopathological analysis revealed significantly reduced alveolar bone loss, and the immunohistochemical staining detected GFP+ fibroblast-like cells and GFP+ osteoblasts within the area of newly formed alveolar bone [40]. In another recent study, Kandalam et al. (2021) investigated the bone regenerative capacity of pre-differentiated GMSCs combined with self-assembling hydrogel scaffold PuraMatrix™ and bone morphogenic protein (BMP2). The maxillary alveolar bone defect was surgically created in athymic rodent models and subsequently filled with GMSCs, which were pre-cultivated in an osteogenic medium for one week. The outcome was evaluated at 4- and 8-weeks post-implantation using microcomputed tomography and histological methods. In comparison with the control group, bone regeneration was significantly enhanced in groups who received pre-differentiated GMSCs treated with BMP2 and seeded on PuraMatrix™ [41].
Another promising stem cell derived from dental tissue is PDLSCs. A periodontal ligament is specialized connective tissue responsible for the regeneration of adjacent periodontal structures. PDLSCs are multipotent cells with high proliferation activity and the capability to differentiate into osteoblasts, cementoblasts, chondroblasts, and adipocytes [42]. Therefore, PDLSCs have also been examined as a possible source for bone regeneration. An in vivo study by Tour et al. (2012) demonstrated the ability of allogenic PDLSCs to form alveolar bone, periodontal ligament, and cementum-like tissue to repair periodontal defects caused by periodontitis [43]. More recently, Iwata et al. (2018) conducted a single-institute clinical study in which the authors created autologous three-layered PDLSC sheets combined with β-tricalcium phosphate bone fillers and transplanted them into the bony defects of 10 patients suffering from chronic periodontitis. The clinical outcomes were evaluated at 3 and 6 months after transplantation. A significant healing process of deep periodontal defects was detected in all cases, including an increase in radiographic bone height and reduction in periodontal probing depth without severe adverse effects. However, the main constraint of this study was the small number of patients. Nevertheless, the “Cell sheet engineering technology” developed by the authors is a promising approach that could be implemented in routine tissue regenerative techniques in dentistry [44].
A relatively newly discovered population of oral stem cells easily collected from surgically removed periapical cysts are hPCy-MSCs, which exhibit similar characteristics to other dental tissue-derived MSCs [45]. Tatullo et al. (2015) drew attention to the advantages of hPCy-MSCs in bone regeneration over the use of DPSCs. According to the qRT-PCR analyses performed, the hPCy-MSCs displayed higher potential to differentiate into osteoblast-like cells, whereas DPSCs tended to give rise to odontoblasts [46]. In the follow-up study, Tatullo et al. (2019) seeded hPCy-MSCs on PLA-based mineral-doped scaffolds to observe their proliferation, viability, and osteogenic/odontogenic gene expression. In all of the investigated parameters, the hPCy-MSCs displayed excellent results involving a high expression of odontogenic/osteogenic marker DMP-1 [47].
There are also several studies that compared bone healing capacities between various types of dental stem cells. For example, Nakajima et al. (2018) examined SHED cells’ osteoinductive potential and mineralization abilities and compared them with human DPSCs and human BM-MSCs. The stem cells seeded on a poly(lactic-co-glycolic acid) barrier membrane were transplanted to an artificial bone defect in the calvaria of an immunodeficient mouse. The histological analyses performed after 12 weeks post-transplantation revealed that SHED formed the largest osteoid area and synthesized more collagen fibers compared with other stem cell types [48]. Furthermore, according to comprehensive meta-analyses of preclinical studies focused on the therapeutic potential of five cell lineages (PDLSCs, BMSCs, DPSCs, GMSCs, and ADSCs) in periodontal tissue regeneration, PDLSCs and BMSCs were the most effective in new alveolar bone, cementum, and periodontal ligament formation [49]. Finally, in the most recent study, Qu et al. (2021) compared the osteogenic potential of four dental-derived MSCs, including DPSCs, PDLSCs, DFSCs, and alveolar bone-derived MSCs (ABMMSCs). Based on the analyses performed, such as osteogenic gene expression and alkaline phosphatase activity staining, ABMMSCs and PDLSCs exhibited higher osteogenic potential in alveolar bone regeneration [28].
Nonetheless, despite the abovementioned auspicious studies, there is undoubtedly a demand for future investigations focused on a better understanding of the biology of dental tissue-derived MSCs.
Table 1.
Regenerative capacity of various dental tissue-derived mesenchymal stem cells (MSCs).
| Type of Dental Tissue-Derived MSCs | In Vivo Models/Human Subjects | Site of Transplantation | Outcome | References |
|---|---|---|---|---|
| DPSCs | Immunocompromised mice | Dorsal surface | Generation of dentine/pulp-like structure | [27] |
| Immunocompromised rats | Subcutaneous site of dorsal surface | Generation of bone tissue with an integral blood supply | [29] | |
| Immunocompromised mice | Subcutaneous site of dorsal surface | Maintenance of MSC characteristics; higher stability compared with PDLSCs in vivo | [7] | |
| 6 patients aged 8 to 12 years old | Unilateral alveolar bone defect | Alveolar bone healing with no ectopic bone formation | [30] | |
| paldSCs | 30 patients | Alveolar bone defect | Improvement in vertical bone augmentation | [19] |
| DFSCs | Immunocompromised rats | Critical-sized calvarial defects | New bone formation | [35] |
| GMSCs | C57BL/6J mice | Second maxillary molar | Reduction in alveolar bone loss and new bone formation | [39] |
| Athymic rodent models | Maxillary alveolar bone defect | Enhanced bone regeneration | [40] | |
| PDLSCs | Immunocompromised rats | Calvarial critical-sized defect | Improvement of bone repair | [42] |
| 10 patients with chronic periodontitis | Root surface of defect site | Healing of deep periodontal defects | [43] | |
| SHED cells | Immunocompromised mice | Calvarial artificial bone defect | Formation of osteoid | [47] |
| SCAP | Minipig model of periodontitis | Local injection in the site of defects | Increased alveolar bone and periodontal tissue regeneration | [23] |
References
- Tetè, G.; D’Orto, B.; Nagni, M.; Agostinacchio, M.; Polizzi, E.; Agliardi, E. Role of induced pluripotent stem cells (IPSCS) in bone tissue regeneration in dentistry: A narrative review. J. Biol. Regul. Homeost. Agents 2021, 34, 1–10.
- Paz, A.G.; Maghaireh, H.; Mangano, F.G. Stem Cells in Dentistry: Types of Intra- and Extraoral Tissue-Derived Stem Cells and Clinical Applications. Stem Cells Int. 2018, 2018, 1–14.
- Fernandez De Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819.
- Zhang, Y.; Wu, D.; Zhao, X.; Pakvasa, M.; Tucker, A.B.; Luo, H.; Qin, K.H.; Hu, D.A.; Wang, E.J.; Li, A.J.; et al. Stem Cell-Friendly Scaffold Biomaterials: Applications for Bone Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 598607.
- Funda, G.; Taschieri, S.; Bruno, G.A.; Grecchi, E.; Paolo, S.; Girolamo, D.; Del Fabbro, M. Nanotechnology Scaffolds for Alveolar Bone Regeneration. Materials 2020, 13, 201.
- Rodriguez, A.-M.; Elabd, C.; Amri, E.-Z.; Ailhaud, G.; Dani, C. The human adipose tissue is a source of multipotent stem cells. Biochimie 2005, 87, 125–128.
- Lei, M.; Li, K.; Li, B.; Gao, L.-N.; Chen, F.-M.; Jin, Y. Mesenchymal stem cell characteristics of dental pulp and periodontal ligament stem cells after in vivo transplantation. Biomaterials 2014, 35, 6332–6343.
- Gan, L.; Liu, Y.; Cui, D.; Pan, Y.; Zheng, L.; Wan, M. Dental Tissue—Derived Human Mesenchymal Stem Cells and Their Potential in Therapeutic Application. Stem Cells Int. 2020, 2020, 8864572.
- Hynes, K.; Menichanin, D.; Bright, R.; Ivanovski, S.; Hutmacher, D.W.; Gronthos, S.; Bartold, P. Induced Pluripotent Stem Cells. J. Dent. Res. 2015, 94, 1508–1515.
- Adamkov, M.; Halašová, E.; Rajcani, J.; Bencat, M.; Výbohová, D.; Rybárová, S.; Galbavy, S. Relation between expression pattern of p53 and survivin in cutaneous basal cell carcinomas. Med. Sci. Monit. 2011, 17, BR74–BR80.
- Phillips, A.M. Overview of the fracture healing cascade. Injury 2005, 36, S5–S7.
- Tsiridis, E.; Upadhyay, N.; Giannoudis, P. Molecular aspects of fracture healing:Which are the important molecules? Injury 2007, 38, S11–S25.
- Huang, G.-J.; Gronthos, S.; Shi, S. Mesenchymal Stem Cells Derived from Dental Tissuesvs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. J. Dent. Res. 2009, 88, 792–806.
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155.
- Tomokiyo, A.; Wada, N.; Maeda, H. Periodontal Ligament Stem Cells: Regenerative Potency in Periodontium. Stem Cells Dev. 2019, 28, 974–985.
- Xu, Q.-C.; Wang, Z.-G.; Ji, Q.-X.; Yu, X.-B.; Xu, X.-Y.; Yuan, C.-Q.; Deng, J.; Yang, P.-S. Systemically transplanted human gingiva-derived mesenchymal stem cells contributing to bone tissue regeneration. Int. J. Clin. Exp. Pathol. 2014, 7, 4922–4929.
- Kim, D.; Lee, A.E.; Xu, Q.; Zhang, Q.; Le, A.D. Gingiva-Derived Mesenchymal Stem Cells: Potential Application in Tissue Engineering and Regenerative Medicine—A Comprehensive Review. Front. Immunol. 2021, 12, 667221.
- Morsczeck, C. Effects of Cellular Senescence on Dental Follicle Cells. Pharmacology 2020, 106, 1–6.
- Grimm, W.D.; Dannan, A.; Giesenhagen, B.; Schau, I.; Varga, G.; Vukovic, M.A.; Sirak, S.V. Translational Research: Palatal-derived Ecto-mesenchymal Stem Cells from Human Palate: A New Hope for Alveolar Bone and Cranio-Facial Bone Reconstruction. Int. J. Stem Cells 2014, 7, 23–29.
- Naung, N.Y.; Duncan, W.; De Silva, R.; Coates, D. Localization and characterization of human palatal periosteum stem cells in serum-free, xeno-free medium for clinical use. Eur. J. Oral Sci. 2019, 127, 99–111.
- Marrelli, M.; Paduano, F.; Tatullo, M. Human Periapical Cyst–Mesenchymal Stem Cells Differentiate Into Neuronal Cells. J. Dent. Res. 2015, 94, 843–852.
- Ayoub, S.; Berbéri, A.; Fayyad-Kazan, M. An update on human periapical cyst-mesenchymal stem cells and their potential applications in regenerative medicine. Mol. Biol. Rep. 2020, 47, 2381–2389.
- Li, G.; Han, N.; Zhang, X.; Yang, H.; Cao, Y.; Wang, S.; Fan, Z. Local Injection of Allogeneic Stem Cells from Apical Papilla Enhanced Periodontal Tissue Regeneration in Minipig Model of Periodontitis. BioMed Res. Int. 2018, 2018, 3960798.
- Spagnuolo, G.; Codispoti, B.; Marrelli, M.; Rengo, C.; Rengo, S.; Tatullo, M. Commitment of Oral-Derived Stem Cells in Dental and Maxillofacial Applications. Dent. J. 2018, 6, 72.
- Andrukhov, O.; Behm, C.; Blufstein, A.; Rausch-Fan, X. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: Implication in disease and tissue regeneration. World J. Stem Cells 2019, 11, 604–617.
- Bajestan, M.N.; Rajan, A.; Edwards, S.P.; Aronovich, S.; Cevidanes, L.H.S.; Polymeri, A.; Travan, S.; Kaigler, D. Stem cell therapy for reconstruction of alveolar cleft and trauma defects in adults: A randomized controlled, clinical trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 793–801.
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and invivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630.
- Qu, G.; Li, Y.; Chen, L.; Chen, Q.; Zou, D.; Yang, C.; Zhou, Q. Comparison of Osteogenic Differentiation Potential of Human Dental-Derived Stem Cells Isolated from Dental Pulp, Periodontal Ligament, Dental Follicle, and Alveolar Bone. Stem Cells Int. 2021, 2021, 6631905.
- D’Aquino, R.; Graziano, A.; Sampaolesi, M.; Laino, G.; Pirozzi, G.; De Rosa, A.; Papaccio, G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: A pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007, 14, 1162–1171.
- Tanikawa, D.Y.S.; Pinheiro, C.C.G.; Almeida, M.C.A.; Oliveira, C.R.G.C.M.; Coudry, R.D.A.; Rocha, D.L.; Bueno, D.F. Deciduous Dental Pulp Stem Cells for Maxillary Alveolar Reconstruction in Cleft Lip and Palate Patients. Stem Cells Int. 2020, 2020, 6234167.
- Paduano, F.; Aiello, E.; Cooper, P.R.; Marrelli, B.; Makeeva, I.; Islam, M.; Spagnuolo, G.; Maged, D.; De Vito, D.; Tatullo, M. A Dedifferentiation Strategy to Enhance the Osteogenic Potential of Dental Derived Stem Cells. Front. Cell Dev. Biol. 2021, 9, 668558.
- Zhang, J.; Ding, H.; Liu, X.; Sheng, Y.; Liu, X.; Jiang, C. Dental Follicle Stem Cells: Tissue Engineering and Immunomodulation. Stem Cells Dev. 2019, 28, 986–994.
- Tsuchiya, S.; Ohshima, S.; Yamakoshi, Y.; Simmer, J.P.; Honda, M.J. Osteogenic Differentiation Capacity of Porcine Dental Follicle Progenitor Cells. Connect. Tissue Res. 2010, 51, 197–207.
- Lucaciu, O.; Soritau, O.; Gheban, D.; Ciuca, D.R.; Virtic, O.; Vulpoi, A.; Dirzu, N.; Campian, R.; Băciuţ, G.; Popa, C.; et al. Dental follicle stem cells in bone regeneration on titanium implants. BMC Biotechnol. 2015, 15, 1–18.
- Bayat, H.; Shahabinejad, H.; Bayat, M.; Shirian, S.; Mohamadnia, A.; Alijani, M.; Godarzi, A.; Shojaei, P.; Shojaei, S.; Shevidi, A.; et al. Osteogenic differentiation of follicular stem cells on nano-Saghez scaffold containing BMP2. J. Orthop. Surg. Res. 2019, 14, 1–12.
- Honda, M.J.; Imaizumi, M.; Suzuki, H.; Ohshima, S.; Tsuchiya, S.; Satomura, K. Stem cells isolated from human dental follicles have osteogenic potential. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 111, 700–708.
- Gao, X. Gingiva-derived Mesenchymal Stem Cells and Their Potential Applications in Oral and Maxillofacial Diseases. Curr. Stem Cell Res. Ther. 2020, 15, 43–53.
- Zhao, N.; Wu, Z.; Qin, L.; Guo, Z.; Li, D. Characteristics and Tissue Regeneration Properties of Gingiva-Derived Mesenchymal Stem Cells. Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 135–144.
- Al-Qadhi, G.; Al-Rai, S.; Hafed, L. The Therapeutic Potential of Inflamed Gingiva-Derived Mesenchymal Stem Cells in Preclinical Studies: A Scoping Review of a Unique Biomedical Waste. Stem Cells Int. 2021, 2021, 6619170.
- Sun, Q.; Nakata, H.; Yamamoto, M.; Kasugai, S.; Kuroda, S. Comparison of gingiva-derived and bone marrow mesenchymal stem cells for osteogenesis. J. Cell. Mol. Med. 2019, 23, 7592–7601.
- Kandalam, U.; Kawai, T.; Ravindran, G.; Brockman, R.; Romero, J.; Munro, M.; Ortiz, J.; Heidari, A.; Thomas, R.; Kuriakose, S.; et al. Predifferentiated Gingival Stem Cell-Induced Bone Regeneration in Rat Alveolar Bone Defect Model. Tissue Eng. Part A 2021, 27, 424–436.
- Queiroz, A.; Albuquerque-Souza, E.; Gasparoni, L.M.; de França, B.N.; Pelissari, C.; Trierveiler, M.; Holzhausen, M. Therapeutic potential of periodontal ligament stem cells. World J. Stem Cells 2021, 13, 605–618.
- Tour, G.; Wendel, M.; Tcacencu, I.; Moll, G. Bone Repair Using Periodontal Ligament Progenitor Cell-seeded Constructs. J. Dent. Res. 2012, 91, 789–794.
- Iwata, T.; Yamato, M.; Washio, K.; Yoshida, T.; Tsumanuma, Y.; Yamada, A.; Onizuka, S.; Izumi, Y.; Ando, T.; Okano, T.; et al. Periodontal regeneration with autologous periodontal ligament-derived cell sheets—A safety and efficacy study in ten patients. Regen. Ther. 2018, 9, 38–44.
- Marrelli, M.; Paduano, F.; Tatullo, M. Cells Isolated from Human Periapical Cysts Express Mesenchymal Stem Cell-like Properties. Int. J. Biol. Sci. 2013, 9, 1070–1078.
- Tatullo, M.; Falisi, G.; Amantea, M.; Rastelli, C.; Paduano, F.; Marrelli, M. Dental pulp stem cells and human periap—Ical cyst mesenchymal stem cells in bone tissue regeneration: Comparison of basal and os—Teogenic differentiated gene expression of a newly discovered mesenchymal stem cell lineage. J. Biol. Regul. Homeost. Agents 2015, 29, 713–718.
- Tatullo, M.; Spagnuolo, G.; Codispoti, B.; Zamparini, F.; Zhang, A.; Degli Esposti, M.; Aparicio, C.; Rengo, C.; Nuzzolese, M.; Manzoli, L.; et al. PLA-Based Mineral—Doped Scaffolds Seeded with Human Periapical Cyst-Derived MSCs: A Promising Tool for Regenerative Healing in Dentistry. Materials 2019, 12, 597.
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M.; et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 876–882.
- Li, Q.; Yang, G.; Li, J.; Ding, M.; Zhou, N.; Dong, H.; Mou, Y. Stem cell therapies for periodontal tissue regeneration: A network meta-analysis of preclinical studies. Stem Cell Res. Ther. 2020, 11, 1–15.
More
