Kynurenine pathway (KP) is the major catabolic route of tryptophan, which generates an important enzyme cofactor (NAD+) and a variety of bioactive metabolites (so-called kynurenines) with immunosuppressive functions or neuroprotective, antioxidant, and toxic properties. It is involved in a variety of physiological processes, especially in conditions associated with immune dysfunction, central nervous system disorders, autoimmunity, infection, diabetes, and cancer. In normal conditions, tryptophan depletion via KP is initiated by the liver enzyme tryptophan 2,3-dioxygenase (TDO) and the extrahepatic enzyme - indoleamine 2,3-dioxygenase (IDO) that contributes minimally to this process (5–10%). The extrahepatic KP becomes quantitatively more significant under conditions of immune activation. KP metabolites are frequently found in biofluids, tissues, and cell-delivered material at low nanomolar or low micromolar concentration levels. However, in disease conditions, abnormal tryptophan metabolism can be accompanied by changes in levels of KP metabolites.
- Kynurenine Pathway
- Electrochemical Sensors
- Voltammetry
- tryptophan metabolites
- kynurenine
1. Kynurenine Ppathway
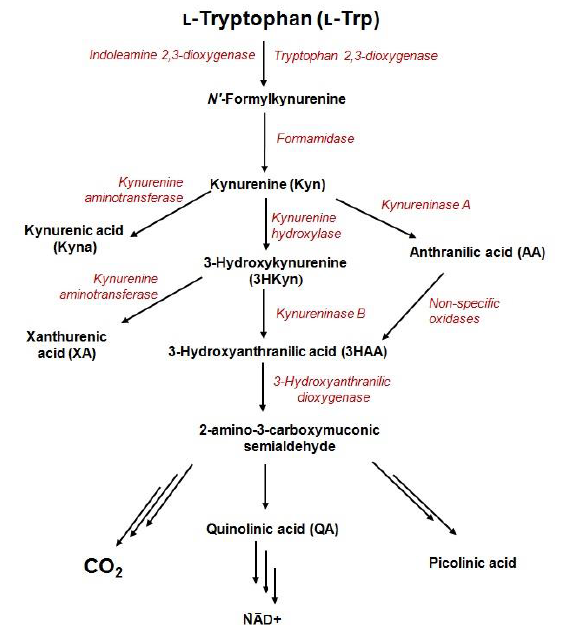
Exploration of the role of tryptophan metabolism provides novel diagnostic and treatment opportunities, however, it requires reliable methods for quantification of its metabolites in a variety of biological samples.
2. Developments in Electrochemical Sensors for the Determination of Kynurenine Pathway Metabolites
| Metabolite | Chemical Formula |
Structure | Eox * [V] (pH = 7.7) |
Isoelectric Point [25] |
|---|---|---|---|---|
| Kyn | C10H12N2O3 | 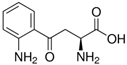 |
0.86 | 6.11 |
| Kyna | C10H7NO3 | 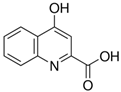 |
1.06 | 2.31 |
| 3HKyn | C10H12N2O4 | 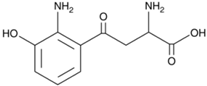 |
0.42 | 6.11 |
| 3HAA | C7H7NO3 | 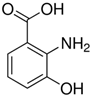 |
0.36 | 3.03 |
| AA | C7H7NO2 | 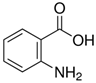 |
0.76 | 3.34 |
| XA | C10H7NO4 | 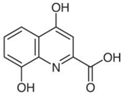 |
0.65 | 3.25 |
| QA | C7H5NO4 | 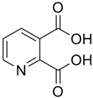 |
n.d. | 2.27 |
Advantages of the GCE and BDDE surface modification have also been emphasized during the design of voltammetric sensors for Kyn [31][32]. Electrochemical deposition of Bi film onto the BDDE surface presents an easy and rapid way to improve the sensor sensitivity toward Kyn measured by DPV and it reaches a low LOD (30 nM) [31]. The coating of a GCE surface with a thin layer of Nafion polymer allows for detection of lower contents of Kyn, as the cationic form of this molecule can be pre-concentrated onto the electrode surface before the stripping step [32]. The Nafion layer can be formed by a drop-coating method (without the need for sophisticated apparatus) and easily removed by polishing using alumina slurries. Furthermore, Kyn can be effectively accumulated onto the Nafion-coated GCE at the potential of +0.5 V in 0.1 M H2SO4, before being stripped by scanning potential toward more positive values [32]. This strategy allows for working with diluted samples and decreases some interferences delivered from the sample matrix components. The applicability of Bi film-modified and Nafion-coated sensors was confirmed for the analysis of material derived from cultures of human cancer cells. Karami et al. have also developed the sensor for Kyn quantification in culture medium collected from cancer cells, but applying a multi-stage modification of the surface of the screen-printed gold electrodes (AuSPEs) [33]. The protocol for the modification of AuSPEs’ surface includes the deposition of carboxylated multiwall carbon nanotubes and immobilization of monoclonal antibody (mAb) specific to Kyn.
3. Interferences
Biological samples are complex media since they contain multiple organic and inorganic components, along with the presence of trace amounts of a target molecule [35]. The overlapping signals from electro-active compounds that have relatively close oxidation potentials pose one of the major problems during electrochemical analysis of a real sample. The careful selection of the electrode support and the measurement conditions allows for improvement of selectivity and sensitivity of electrochemical measurements [31]. Regarding the electrochemical determination of KP metabolites, special attention should be paid to the following issues:- Influence of Trp on Kyn and AA signals;
- Overlapping signals from 3HKyn and 3HAA;
- Interferences delivered by tryptophan metabolites formed in other pathways;
- Effect of amino acids;
- Interferences from uric acid, ascorbic acid, and dopamine.
References
- Takikawa, O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated l-tryptophan metabolism. Biochem. Biophys. Res. Commun. 2005, 338, 12–19.
- Marszalek-Grabska, M.; Walczak, K.; Gawel, K.; Wicha-Komsta, K.; Wnorowska, S.; Wnorowski, A.; Turski, W.A. Kynurenine emerges from the shadows–Current knowledge on its fate and function. Pharmacol. Ther. 2021, 225, 107845–107872.
- Badawy, A.; Namboodiri, A.M.; Moffett, J.R. The end of the road for the tryptophan depletion concept in pregnancy and infection. Clin. Sci. 2016, 130, 1327–1333.
- Mellor, A.L.; Lemos, H.; Huang, L. Indoleamine 2,3-Dioxygenase and Tolerance: Where Are We Now? Front. Immunol. 2017, 8, 1–8.
- Badawy, A.A.B. Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int. J. Tryptophan Res. 2017, 10, 1–10.
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794.
- Sinclair, L.V.; Neyens, D.; Ramsay, G.; Taylor, M.P.; Cantrell, D.A. Single cell analysis of kynurenine and System L amino acid transport in T cells. Nat. Commun. 2018, 9, 1–11.
- Novikov, O.; Wang, Z.; Stanford, E.A.; Parks, A.J.; Ramirez-Cardenas, A.; Landesman, E.; Laklouk, I.; Sarita-Reyes, C.; Gusenleitner, D.; Li, A.; et al. An Aryl Hydrocarbon Receptor-Mediated Amplification Loop That Enforces Cell Migration in ER-/PR-/Her2- Human Breast Cancer Cells. Mol. Pharmacol. 2016, 90, 674–688.
- Bock, K.W. Aryl hydrocarbon receptor (AHR) functions: Balancing opposing processes including inflammatory reactions. Biochem. Pharmacol. 2020, 178, 114093.
- Turski, M.P.; Turska, M.; Paluszkiewicz, P.; Parada-Turska, J.; Oxenkrug, G.F. Kynurenic Acid in the Digestive system—new Facts, new challenges. Int. J. Tryptophan Res. 2013, 6, 47–55.
- Morita, T.; Saito, K.; Takemura, M. 3-Hydroxyanthranilic acid, an L-tryptophan metabolite, induces apoptosis in monocyte-derived cells stimulated by interferon-γ. Ann. Clin. Biochem. 2001, 38, 242–251.
- Zaher, S.S.; Germain, C.; Fu, H.; Larkin, D.F.P.; George, A.J.T. 3-Hydroxykynurenine Suppresses CD4+ T-Cell Proliferation, Induces T-Regulatory-Cell Development, and Prolongs Corneal Allograft Survival. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2640–2648.
- Guillemin, G.J. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012, 279, 1325–1365.
- Malina, H.; Richter, C.; Frueh, B.; Hess, O.M. Lens epithelial cell apoptosis and intracellular Ca2+ increasein the presence of xanthurenic acid. BMC Ophthalmol. 2002, 2, 1–7.
- Dhakar, N.K.; Caldera, F.; Bessone, F.; Cecone, C.; Pedrazzo, A.R.; Cavalli, R.; Dianzani, C.; Trotta, F. Evaluation of solubility enhancement, antioxidant activity, and cytotoxicity studies of kynurenic acid loaded cyclodextrin nanosponge. Carbohydr. Polym. 2019, 224, 115168–115176.
- Chobot, V.; Hadacek, F.; Weckwerth, W.; Kubicova, L. Iron chelation and redox chemistry of anthranilic acid and 3-hydroxyanthranilic acid: A comparison of two structurally related kynurenine pathway metabolites to obtain improved insights into their potential role in neurological disease development. J. Organomet. Chem. 2015, 782, 103–110.
- Sathyasaikumar, K.V.; Tararina, M.; Wu, H.Q.; Neale, S.A.; Weisz, F.; Salt, T.E.; Schwarcz, R. Xanthurenic Acid Formation from 3-Hydroxykynurenine in the Mammalian Brain: Neurochemical Characterization and Physiological Effects. Neuroscience 2017, 367, 85–97.
- Li, G.; Miao, P. Theoretical Background of Electrochemical Analysis. In Electrochemical Analysis of Proteins and Cells. SpringerBriefs in Molecular Science; Springer: Berlin, Germany, 2013; pp. 5–18.
- Kato, D.; Kamata, T.; Sumimoto, M. Electrochemical Detection of Tryptophan Metabolites via Kynurenine Pathway by Using Nanocarbon Films. Electroanalysis 2021.
- Muzyka, K.; Sun, J.; Fereja, T.H.; Lan, Y.; Zhang, W.; Xu, G. Boron-doped diamond: Current progress and challenges in view of electroanalytical applications. Anal. Methods 2019, 11, 397–414.
- Sadok, I.; Tyszczuk-Rotko, K.; Mroczka, R.; Staniszewska, M. Simultaneous voltammetric analysis of tryptophan and kynurenine in culture medium from human cancer cells. Talanta 2020, 209, 120574–120584.
- Sadok, I.; Jędruchniewicz, K.; Rawicz-Pruszyński, K.; Staniszewska, M. UHPLC-ESI-MS/MS Quantification of Relevant Substrates and Metabolites of the Kynurenine Pathway Present in Serum and Peritoneal Fluid from Gastric Cancer Patients—Method Development and Validation. Int. J. Mol. Sci. 2021, 22, 6972.
- Malone, M.A.; Zuo, H.; Lunte, S.M.; Smyth, M.R. Determination of tryptophan and kynurenine in brain microdialysis samples by capillary electrophoresis with electrochemical detection. J. Chromatogr. A 1995, 700, 73–80.
- Bizzarri, M.; Catizone, A.; Pompei, M.; Chiappini, L.; Curini, L.; Lagana, A. Determination of urinary tryptophan and its metabolites along the nicotinic acid pathway by high performance liquid chromatography with ultraviolet detection. Biomed. Chromatogr. 1990, 4, 24–27.
- Tömösi, F.; Kecskeméti, G.; Cseh, E.K.; Szabó, E.; Rajda, C.; Kormány, R.; Szabó, Z.; Vécsei, L.; Janáky, T. A validated UHPLC-MS method for tryptophan metabolites: Application in the diagnosis of multiple sclerosis. J. Pharm. Biomed. Anal. 2020, 185, 113246–113257.
- Badawy, A.A.-B.; Morgan, C.J. Rapid Isocratic Liquid Chromatographic Separation and Quantification of Tryptophan and Six kynurenine Metabolites in Biological Samples with Ultraviolet and Fluorimetric Detection. Int. J. Tryptophan Res. 2010, 3, 175–186.
- Singh, R.; Kashyap, S.; Kumar, S.; Abraham, S.; Gupta, T.K.; Kayastha, A.M.; Malhotra, B.D.; Saxena, P.S.; Srivastava, A.; Singh, R.K. Excellent storage stability and sensitive detection of neurotoxin quinolinic acid. Biosens. Bioelectron. 2017, 90, 224–229.
- Kubicova, L.; Hadacek, F.; Bachmann, G.; Weckwerth, W.; Chobot, V. Coordination Complex Formation and Redox Properties of Kynurenic and Xanthurenic Acid Can A ect Brain Tissue Homeodynamics. Antioxidants 2019, 8, 476.
- Tajik, S.; Beitollahi, H.; Mohammadi, S.Z.; Azimzadeh, M.; Zhang, K.; Van Le, Q.; Yamauchi, Y.; Jang, H.W.; Shokouhimehr, M. Recent developments in electrochemical sensors for detecting hydrazine with different modified electrodes. RSC Adv. 2020, 10, 30481–30498.
- Marrugo-Ramírez, J.; Rodríguez-Núñez, M.; Marco, M.-P.; Mir, M.; Samitier, J. Kynurenic Acid Electrochemical Immunosensor: Blood-Based Diagnosis of Alzheimer’s Disease. Biosensors 2021, 11, 20.
- Ilona Sadok; Katarzyna Tyszczuk-Rotko; Robert Mroczka; Magdalena Staniszewska; Simultaneous voltammetric analysis of tryptophan and kynurenine in culture medium from human cancer cells. Talanta 2019, 209, 120574, 10.1016/j.talanta.2019.120574.
- Ilona Sadok; Katarzyna Tyszczuk-Rotko; Robert Mroczka; Jędrzej Kozak; Magdalena Staniszewska; Improved Voltammetric Determination of Kynurenine at the Nafion Covered Glassy Carbon Electrode – Application in Samples Delivered from Human Cancer Cells. International Journal of Tryptophan Research 2021, 14, 1-14, 10.1177/11786469211023468.
- Pari Karami; Mir Reza Majidi; Mohammad Johari-Ahar; Jaleh Barar; Yadollah Omidi; Development of screen-printed tryptophan-kynurenine immunosensor for in vitro assay of kynurenine-mediated immunosuppression effect of cancer cells on activated T-cells. Biosensors and Bioelectronics 2017, 92, 287-293, 10.1016/j.bios.2016.11.010.
- Chun-Yueh Huang; Danny O'Hare; I-Jen Chao; Hung-Wei Wei; Yi-Fan Liang; Bin-Da Liu; Mei-Hwa Lee; Hung-Yin Lin; Integrated potentiostat for electrochemical sensing of urinary 3-hydroxyanthranilic acid with molecularly imprinted poly(ethylene-co-vinyl alcohol). Biosensors and Bioelectronics 2015, 67, 208-213, 10.1016/j.bios.2014.08.018.
- Beshare Hashemi; Parvin Zohrabi; Mojtaba Shamsipur; Recent developments and applications of different sorbents for SPE and SPME from biological samples. Talanta 2018, 187, 337-347, 10.1016/j.talanta.2018.05.053.
