Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ke-Vin Chang and Version 2 by Conner Chen.
Sarcopenia, defined as a decline in muscle mass and function related to aging, affects both limb and swallowing-related muscles. Sarcopenic dysphagia is characterized by decreased swallowing function; therefore, early detection of subclinical dysphagia and subsequent intervention appear to be crucial in the elderly.
- sarcopenia
1. Sarcopenia
Sarcopenia is a clinical syndrome that refers to a gradual and generalized loss of skeletal muscle mass and strength [1][2]. The decline of muscle mass is about 3–8% per decade after the age of 30 years and rises to 15% after 70 years of age [2][3][6,7]. Recently, the concept of the gut-muscle axis has been proposed to play a crucial role in the development of sarcopenia. The gut microbiota has been found to impact muscle volume and performance through mediating inflammatory reactions, immunity, endocrine function, and energy metabolism [4][5][8,9]. According to the European Working Group on Sarcopenia in Older People (EWGSOP) [6][1] and Asian Working Group for Sarcopenia (AWGS) [7][10] diagnostic criteria, sarcopenia is defined as low muscle mass, strength, and/or physical performance. The cut-point value of sarcopenia revised by the European Working Group on Sarcopenia in Older People (EWGSOP) in 2018 is (1) <7.0 kg/m2 in men and <5.5 kg/m2 in women for low muscle quantity; (2) handgrip strength < 27 kg for men and < 16 kg for women for low muscle strength; (3) 6-m walk < 0.8 m/s, Short Physical Performance Battery score ≤ 8, 5-time chair stand test ≥ 15 s, timed up-and-go test ≥ 20 s, or 400-m walk ≥ 6 min for low physical performance [8][11]. Moreover, the cut-point value suggested by Asian Working Group for Sarcopenia (AWGS) 2019 consensus is: (1) <7.0 kg/m2 in men and <5.4 kg/m2 in women on dual-energy X-ray absorptiometry; and <7.0 kg/m2 in men and <5.7 kg/m2 in women on bioimpedance analysis; (2) handgrip strength < 28 kg for men and < 18 kg for women for low muscle strength; (3) 6-m walk < 1.0 m/s, Short Physical Performance Battery score ≤ 9, or 5-time chair stand test ≥ 12 s for low physical performance [9][12]. Due to the variations of its diagnostic criteria, the prevalence of sarcopenia ranges between 1% and 29% in the elderly [10][13]. Of note, sarcopenia may also reduce the strength of swallowing muscles, leading to dysphagia [11][12][14,15].
2. Dysphagia
Dysphagia is defined as difficulty in eating and swallowing [13][16], presenting with impaired or prolonged transit of food or liquids from the oral cavity to the esophagus [14][17]. It commonly ensues as a consequence of diseases like esophageal cancer, stroke, and Parkinson’s disease [15][18]. The swallowing process can be divided into the following four stages: the oral preparatory, oral, pharyngeal, and esophageal phases. The dysfunction in any of the aforementioned stages can cause dysphagia [16][19]. The association of dysphagia with several adverse health outcomes—e.g., malnutrition, dehydration, respiratory infections, aspiration pneumonia, increased readmissions, institutionalization, and mortality-have been identified [14][17]. As such, early detection of subclinical dysphagia and subsequent intervention are crucial in the elderly.
3. Sarcopenic Dysphagia
Dysphagia caused by sarcopenia is categorized as sarcopenic dysphagia [17][3]. Indisputably, dysphagia is also a risk factor for malnutrition in older patients. The mechanism behind sarcopenic dysphagia is thought to be a decline in the swallowing-related muscle mass and strength. Age-related loss of swallowing muscle mass can be manifested as a decrease in the thickness of the tongue [18][20], geniohyoid muscle [19][21], and pharyngeal wall, and an increase in the pharyngeal lumen size [20][22]. These changes contribute to decreased tongue strength, reduced range of tongue motion, weakened pharyngeal muscle contraction, and deteriorated endurance of swallowing muscles, all of which are the risk factors of dysphagia [21][23].
Dysphagia increases the risk of malnutrition due to reduced oral intake. Patients who can achieve full oral intake without additional nutrition support through parenteral routes are able to obtain higher energy contents from food than those who cannot [22][24]. Decreased nutrition support also leads to weight loss and disrupted synthesis of skeletal muscles, which consequently result in further development of sarcopenia. Therefore, a vicious cycle of sarcopenia and dysphagia eventually becomes inevitable (Figure 1).
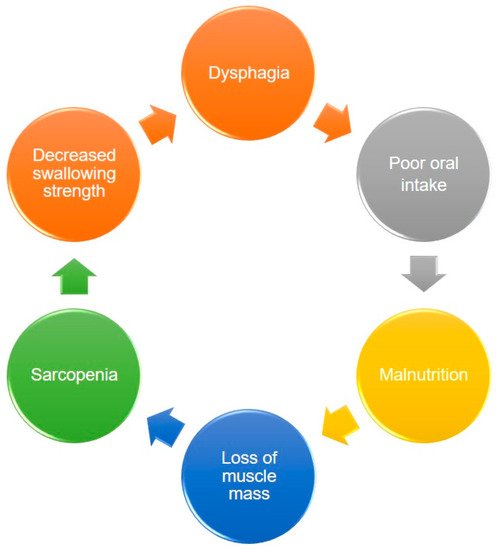
Figure 1. The vicious circle between dysphagia, malnutrition, and sarcopenia.
The consensus on the diagnosis of sarcopenic dysphagia has been established at the 19th Annual Meeting of the Japanese Society of Dysphagia Rehabilitation [21][23]. The coexistence of dysphagia and sarcopenia is mandatory for the diagnosis. In other words, if the main cause of dysphagia is sarcopenia accompanied by loss of swallowing muscle mass identified on imaging modalities, ‘definite sarcopenic dysphagia’ is confirmed. If sarcopenia could not be ruled out as a cause of dysphagia, ‘probable sarcopenic dysphagia’ is considered. If the main cause of dysphagia is sarcopenia with the coexistence of diseases that may be linked to dysphagia (such as stroke or head/neck cancer), ‘possible sarcopenic dysphagia’ is defined (Table 1) [23][25].
Table 1. Diagnostic criteria for sarcopenic dysphagia.
| Item | Criteria |
|---|---|
| 1 | Presence of dysphagia |
| 2 | Presence of whole-body sarcopenia |
| 3 | The results of imaging tests (computed tomography, magnetic resonance imaging, ultrasonography) are consistent with loss of swallowing muscle mass |
| 4 | The causes of dysphagia are excluded except for sarcopenia |
| 5 | The main cause of dysphagia is considered to be sarcopenia |
Definite diagnosis: 1, 2, 3, 4. Probable diagnosis: 1, 2, 4. Possible diagnosis: 1, 2, 5.
A 5-step diagnostic algorithm has been developed by the Working Group on Sarcopenic Dysphagia [21][23] (Figure 2). It categorizes the examinees into three groups: probable sarcopenic dysphagia, possible sarcopenic dysphagia, and no sarcopenic dysphagia. Patients with diseases (other than sarcopenia) directly leading to dysphagia are excluded. Compared with the aforementioned initial consensus, the advantage of this diagnostic algorithm is the use of tongue pressure to represent its strength without assessing swallowing muscle mass. The cut-off value for defining low tongue pressure is 20 kPa. In 2017, Mori et al. [23][25] enrolled 119 old inpatients for verifying the reliability and validity of the 5-step algorithm. The intra-class coefficients for intra- and inter-rater reliability were 0.87 (95% confidence interval [CI]: 0.73–1.01), and 0.98 (95% CI: 0.92–1.02), respectively. In 2019, Wakabayashi et al. [24][26] recruited 108 patients to assess the prevalence and prognosis of sarcopenic dysphagia by using the same algorithm. The study revealed the prevalence of sarcopenic dysphagia as 32% in patients who require rehabilitation for swallowing dysfunction. The authors also identified that the swallowing function at discharge was worse in patients with sarcopenic dysphagia vs. non-sarcopenic dysphagia.
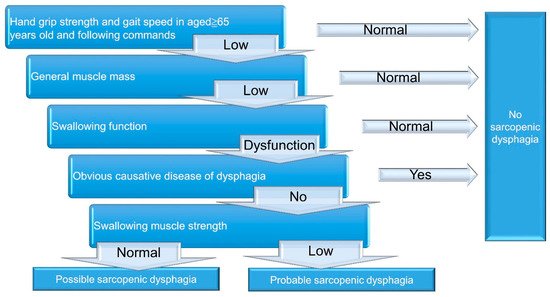
Figure 2. A 5-step diagnostic algorithm for the diagnosis of sarcopenic dysphagia.
