Multilevel alterations of hippocampal function have been identified as a common denominator of memory impairments in a number of psychiatric and neurodegenerative diseases. For many years, the glutamatergic and cholinergic systems have been the main targets of therapeutic treatments against these symptoms. Type 4 serotonin receptors (5-HT4Rs) is a new therapeutic target for memory disorders. To date, much of the researched information gathered by scientists from both animal models and humans converge on pro-mnesic and anti-amnesic properties of 5-HT4Rs activation.
- 5-HT4Rs
- serotonin
- hippocampus
- memory disorders
- therapeutic target
- synaptic plasticity
- cognition
1. Introduction
2. Episodic Memory Function and the Hippocampal Formation
From the second half of the 20th century, case studies of patients with amnesia, as well as the development of a large number of animal models with memory disorders, enabled major breakthroughs in the understanding of the brain memory system—or how the brain stores different kinds of information. The idea of the existence of different forms of memory stems from this wealth of clinical work and fundamental studies. Often viewed as the most sophisticated, episodic memory is characterized by the capacity to re-experience a past personal event, situation or experience in the context in which it originally occurred [8]. A characteristic feature of episodic memory resides in the ability to bind together various interrelated stimuli and their spatial, temporal and conceptual relationships, to build up coherent memory representations [9]. Unfortunately, episodic memory shows the largest degree of decline in age-related cognitive impairments such as in AD [1] or even in several psychiatric contexts, such as MDD [3].2.1. The Hippocampal Formation
2.2. The Hippocampal Formation Circuitry
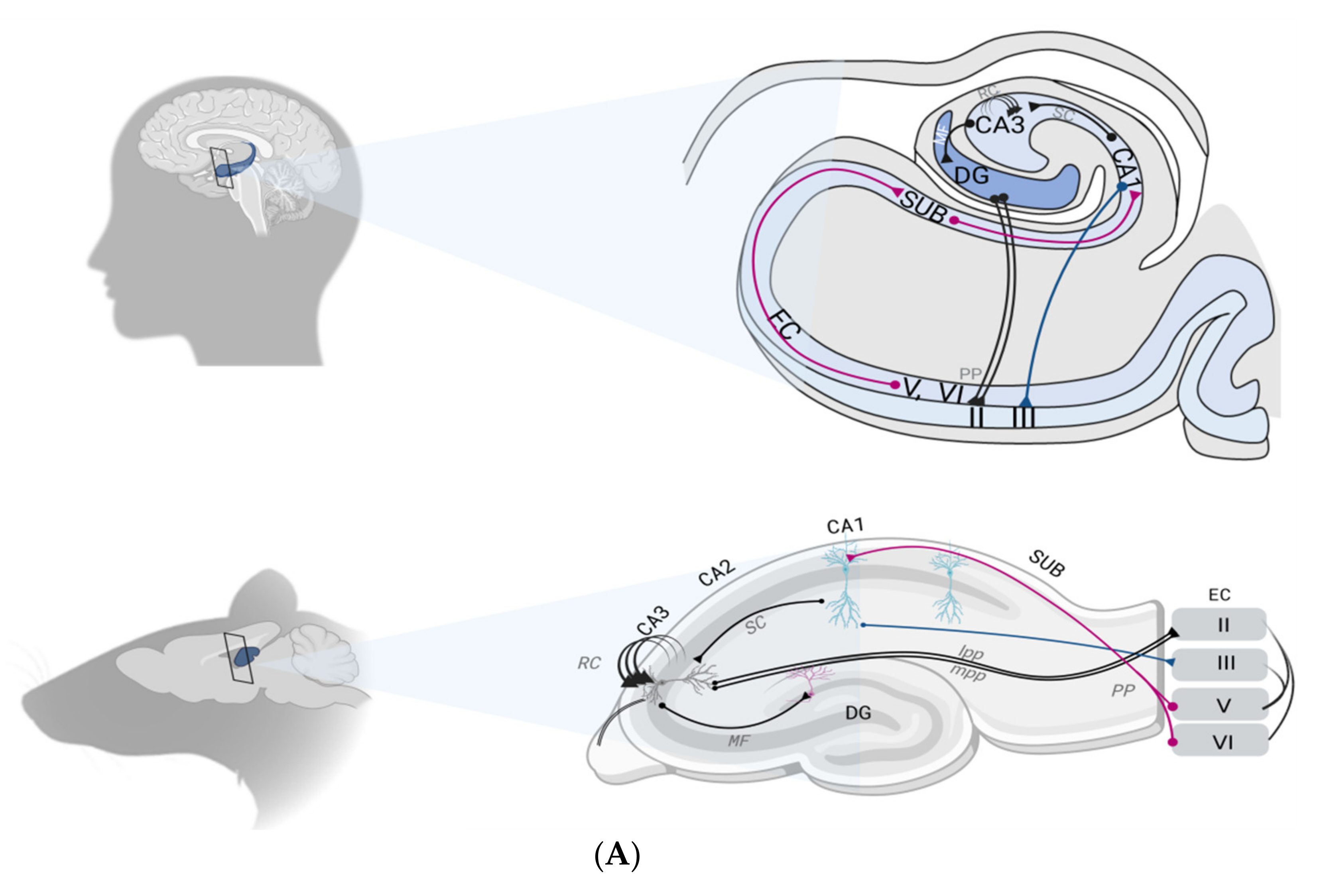
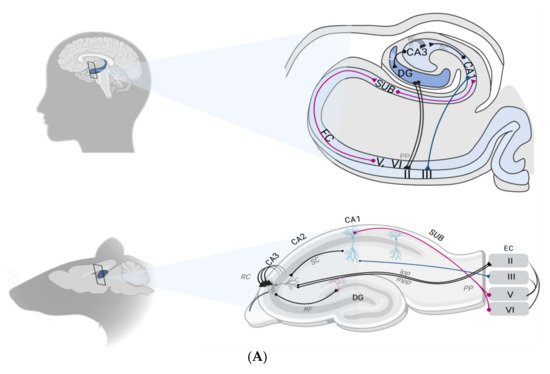
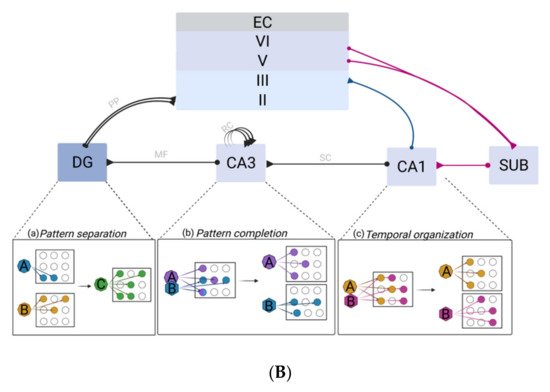

2.3. Synaptic Plasticity as a Correlate of Hippocampal Memory
2.4. Neurotransmission Systems in the Hippocampus
3. Relevance of 5-HT4Rs Modulation in Memory Disorders
5-HT4Rs belong to excitatory Gαs (stimulatory alpha subunit) protein-coupled receptors (GPCR). Their activation exerts a stimulatory effect through the activation of adenylate cyclase (ADC) as a primary mode of signal transduction on cAMP concentration. This second messenger interacts with various other proteins including PKA, which is known to modulate the activation of gene expression modifying transcription factors, such as the cAMP response element-binding protein (CREB) [38]. Additionally, an intriguing aspect of metabotropic 5-HTRs is their ability to elicit non-canonical pathways that can be G-protein independent. With regard to 5-HT4Rs, their activation can initiate phosphorylation of their associated non-receptor tyrosine kinase Src, which activates mitogen-activated protein kinases (MAPK) including the extracellular signal-regulated kinases (ERK) [38][39]. Quite interestingly, these molecular actors also appear to be involved in LTP. Moreover, cAMP/signaling and BDNF expression were found to be disrupted in a number of animal models of neurological disorders [40][41] and found to be enhanced after 5-HT4Rs activation [40][41] (Figure 2). Altogether, this raises the interest of 5-HT4Rs-targeting in plasticity-related memory enhancement.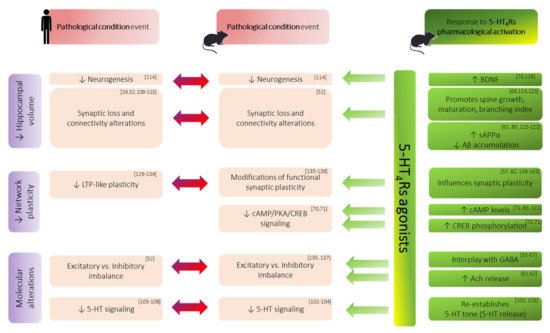
3.1. Insights from Animal Behavior Investigations
3.2. Distribution of 5-HT4Rs in CNS and Memory Disorders
3.3. Morphological/Structural Alterations of Hippocampal Formation in Memory Disorders
3.4. Functional Synaptic Plasticity Impairments
|
Method |
Hippocampal Area |
Plasticity |
Conditioning Stimulus |
5-HT4Rs Agonist |
Effects of 5-HT4Rs Activation on Plasticity |
Reference |
|---|---|---|---|---|---|---|
|
In vivo |
DG |
LTP |
HFS (200 Hz) |
RS67333 |
↓ |
Kulla and Manahan-Vaughan.2002 |
|
LTP |
HFS (200 Hz) |
5-Methoxytryptamine |
= |
|||
|
LTP |
HFS (10 × 400 Hz) |
RS67333 |
Transient ↑ and curtailed |
Marchetti et al. 2004 |
||
|
LTP |
HFS (200 Hz) |
RS67333 |
Curtailed |
Twarkowski et al. 2016 |
||
|
DP |
LFS (5 Hz) |
RS67333 |
Blocked |
|||
|
LTD |
LFS (1 Hz) |
RS67633 |
↓ |
|||
|
CA3 |
LTP |
HFS (4 × 100 Hz) |
RS67333 |
↓ |
Twarkowski et al. 2016 |
|
|
LTD |
LFS (1 Hz) |
RS67333 |
↓ |
|||
|
CA1 |
LTP |
HFS (5 × 400 Hz) |
SC53116 |
↑ |
Matsumoto et al. 2001 |
|
|
LTP |
HFS (4 × 100 Hz) |
RS67333 |
= |
Kemp and Manahan-Vaughan 2005 |
||
|
LTD |
LFS (1 Hz) |
RS67333 |
↓ |
|||
|
Ex vivo |
CA1 |
LTP |
HFS (1 × 100 Hz) |
RS67333 |
= |
Lecouflet et al. 2020 |
|
LTP |
TBS (4 × 5 Hz) |
RS67333 |
↓ |
|||
|
SUB |
LTP |
HFS (4 × 100 Hz) |
RS67333 |
= |
||
|
LTD |
LFS (1 Hz) |
RS67333 |
↑ |
Wawra et al. 2014 |
These data support the fact that 5-HT4Rs, through their modulatory effects on synaptic plasticity processes, will enable the hippocampus to ensure its filtering role of information during acquisition and more variable changes in the downstream areas.This perspective seems consistent with clinical data that suggest that an increased signal-to-noise ratio within the hippocampus improves the encoding accuracy, a function which is thought to be mainly supported by the DG where 5-HT4Rs are most abundantly expressed [100].
4. Concluding Remarks and Future Perspectives
Overall, arguments to consider 5-HT4Rs as a target of choice for the treatment of memory impairments mainly stem from preclinical evidence. In fact, only a few experiments were performed on humans.
Nonetheless, quite recently, SUVN-D4010, a novel, potent, highly selective 5-HT4Rs partial agonist intended for the treatment of cognitive disorders, was found to be safe and well tolerated in healthy human subjects, even in elderly population (Suven Life Sciences, NCT02575482 and NCT03031574). Lastly, the results published last year regarding prucalopride are also of high interest. Indeed, while already approved by the FDA in 2018 to treat chronic idiopathic constipation, prucalopride was investigated in a battery of cognitive tests related to hippocampal functions. In healthy human subjects, prucalopride showed beneficial effects on learning and memory performance (NCT03572790) [100] and is currently under investigation for its role in depression. Evidence for improved memory performance after 5-HT4Rs activation in humans was extended by a very recent fMRI study. Following prucalopride intake, hippocampal activity during memory recall was significantly increased compared with volunteers receiving a placebo [101].
The use of 5-HT4Rs ligands in the treatment of memory deficits is still an ongoing challenge but has long been—and still unfortunately is—restricted to AD and MDD. However, a number of functional and morphological changes within the hippocampus are a common denominator of a broader range of both normal ageing and neurological diseases (such as PD, MDD, SCZ). A large amount of data from both animal models and humans have now reached a consensus on the fact that 5-HT4Rs activation can attenuate some of these hippocampal dysfunctions. This ultimately raises the exciting potential of restoring—or at least limiting—memory decline in these pathologies. Nevertheless, a deeper understanding of the mechanisms at work is still needed and would help further development. In this view, studies that investigate 5-HT4Rs effects on hippocampal function in a more integrated view should provide substantial insights.
References
- El Haj, M.; Roche, J.; Gallouj, K.; Gandolphe, M.-C. Autobiographical Memory Compromise in Alzheimer’s Disease: A Cognitive and Clinical Overview. Gériatrie Psychol. Neuropsychiatr. Viellissement 2017, 15, 443–451.
- Das, T.; Hwang, J.J.; Poston, K.L. Episodic Recognition Memory and the Hippocampus in Parkinson’s Disease: A Review. Cortex 2019, 113, 191–209.
- Darcet, F.; Gardier, A.; Gaillard, R.; David, D.; Guilloux, J.-P. Cognitive Dysfunction in Major Depressive Disorder. A Translational Review in Animal Models of the Disease. Pharmaceuticals 2016, 9, 9.
- Guo, J.Y.; Ragland, J.D.; Carter, C.S. Memory and Cognition in Schizophrenia. Mol. Psychiatry 2019, 24, 633–642.
- Klein, S.B.; Nichols, S. Memory and the Sense of Personal Identity. Mind 2012, 121, 677–702.
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, L.; Winblad, B.; et al. Clinical Trials and Late-Stage Drug Development for Alzheimer’s Disease: An Appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283.
- FDA. FDA Grants Accelerated Approval for Alzheimer’s Drug. 2021. Available online: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug (accessed on 13 July 2021).
- Squire, L.R. Memory Systems of the Brain: A Brief History and Current Perspective. Neurobiol. Learn. Mem. 2004, 82, 171–177.
- Tulving, E.; Markowitsch, H.J. Episodic and Declarative Memory: Role of the Hippocampus. Hippocampus 1998, 8, 198–204.
- Scoville, B.S.; Milner, B. Loss of Recent Memory after Bilateral Hippocampal Lesions. J. Neurol. Neurosurg. Psychiat. 1957, 20, 11.
- Stein, D.G.; Kimble, D.P. Effects of Hippocampal Lesions and Posttrial Strychnine Administration on Maze Behavior in the Rat. J. Comp. Physiol. Psychol. 1966, 62, 243–249.
- Thompson, R.; Langer, S.K.; Rich, I. Lesions of the limbic system and short-term memory in albino rats. Brain 1964, 87, 537–542.
- Zola, S.M.; Squire, L.R.; Teng, E.; Stefanacci, L.; Buffalo, E.A.; Clark, R.E. Impaired Recognition Memory in Monkeys after Damage Limited to the Hippocampal Region. J. Neurosci. 2000, 20, 451–463.
- Zola-Morgan, S.; Squirre, L.R.; Amaral, G. Human Amnesia and the Medial Temporal Region: Impairment Following a Bilateral Lesion Limited to Field CA1 of the Hippocampus. J. Neurosci. 1986, 6, 2950–2967.
- Eichenbaum, H. Hippocampus. Neuron 2004, 44, 109–120.
- O’Keefe, J.; Nadel, L. The Hippocampus as a Cognitive Map; Clarendon Press: Oxford, UK, 1978; ISBN 978-0-19-857206-0.
- Fanselow, M.S.; Dong, H.-W. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron 2010, 65, 7–19.
- Bannerman, D.M.; Rawlins, J.N.P.; McHugh, S.B.; Deacon, R.M.J.; Yee, B.K.; Bast, T.; Zhang, W.-N.; Pothuizen, H.H.J.; Feldon, J. Regional Dissociations within the Hippocampus—Memory and Anxiety. Neurosci. Biobehav. Rev. 2004, 28, 273–283.
- Moser, M.B.; Moser, E.I. Functional Differentiation in the Hippocampus. Hippocampus 1998, 8, 608–619.
- O’Keefe, J.; Recce, M.L. Phase Relationship between Hippocampal Place Units and the EEG Theta Rhythm. Hippocampus 1993, 3, 317–330.
- Kesner, R.P.; Rolls, E.T. A Computational Theory of Hippocampal Function, and Tests of the Theory: New Developments. Neurosci. Biobehav. Rev. 2015, 48, 92–147.
- Martin, S.J.; Grimwood, P.D.; Morris, R.G.M. Synaptic Plasticity and Memory: An Evaluation of the Hypothesis. Annu. Rev. Neurosci. 2000, 23, 649–711.
- Hebb, D.O. The Organization of Behavior: A Neuropsychological Theory; Wiley: Oxford, UK, 1949.
- Bliss, T.V.P.; Collingridge, G.L. A Synaptic Model of Memory: Long-Term Potentiation in the Hippocampus. Nature 1993, 361, 31–39.
- Bliss, T.V.P.; Lømo, T. Long-Lasting Potentiation of Synaptic Transmission in the Dentate Area of the Anaesthetized Rabbit Following Stimulation of the Perforant Path. J. Physiol. 1973, 232, 331–356.
- Nicoll, R.A. A Brief History of Long-Term Potentiation. Neuron 2017, 93, 281–290.
- Larson, J.; Munkácsy, E. Theta-Burst LTP. Brain Res. 2015, 1621, 38–50.
- Bragin, A.; Jando, G.; Nadasdy, Z.; Hetke, J.; Wise, K.; Buzsaki, G. Gamma (40-100 Hz) Oscillation in the Hippocampus of the Behaving Rat. J. Neurosci. 1995, 15, 47–60.
- Tort, A.B.L.; Kramer, M.A.; Thorn, C.; Gibson, D.J.; Kubota, Y.; Graybiel, A.M.; Kopell, N.J. Dynamic Cross-Frequency Couplings of Local Field Potential Oscillations in Rat Striatum and Hippocampus during Performance of a T-Maze Task. Proc. Natl. Acad. Sci. USA 2008, 105, 20517–20522.
- Nuñez, A.; Buño, W. The Theta Rhythm of the Hippocampus: From Neuronal and Circuit Mechanisms to Behavior. Front. Cell. Neurosci. 2021, 15, 649262.
- Morris, R.G.M.; Anderson, E.; Lynch, G.S.; Baudry, M. Selective Impairment of Learning and Blockade of Long-Term Potentiation by an N-Methyl-D-Aspartate Receptor Antagonist, AP5. Nature 1986, 319, 774–776.
- Davis, S.; Butcher, S.P.; Morris, R.G. The NMDA Receptor Antagonist D-2-Amino-5-Phosphonopentanoate (D-AP5) Impairs Spatial Learning and LTP in Vivo at Intracerebral Concentrations Comparable to Those That Block LTP in Vitro. J. Neurosci. 1992, 12, 21–34.
- Lynch, M.A. Long-Term Potentiation and Memory. Physiol. Rev. 2004, 84, 87–136.
- Cooke, S.F. Plasticity in the Human Central Nervous System. Brain 2006, 129, 1659–1673.
- Kemp, A.; Manahan-Vaughan, D. Hippocampal Long-Term Depression: Master or Minion in Declarative Memory Processes? Trends Neurosci. 2007, 30, 111–118.
- Bliss, T.V.P.; Cooke, S.F. Long-Term Potentiation and Long-Term Depression: A Clinical Perspective. Clinics 2011, 66, 3–17.
- Myhrer, T. Neurotransmitter Systems Involved in Learning and Memory in the Rat: A Meta-Analysis Based on Studies of Four Behavioral Tasks. Brain Res. Rev. 2003, 41, 268–287.
- Bockaert, J.; Claeysen, S.; Compan, V.; Dumuis, A. 5-HT4 Receptors: History, Molecular Pharmacology and Brain Functions. Neuropharmacology 2008, 55, 922–931.
- Restivo, L.; Roman, F.; Dumuis, A.; Bockaert, J.; Marchetti, E.; Ammassari-Teule, M. The Promnesic Effect of G-Protein-Coupled 5-HT4 Receptors Activation Is Mediated by a Potentiation of Learning-Induced Spine Growth in the Mouse Hippocampus. Neuropsychopharmacology 2008, 33, 2427–2434.
- Pascual-Brazo, J.; Castro, E.; Díaz, Á.; Valdizán, E.M.; Pilar-Cuéllar, F.; Vidal, R.; Treceño, B.; Pazos, Á. Modulation of Neuroplasticity Pathways and Antidepressant-like Behavioural Responses Following the Short-Term (3 and 7 Days) Administration of the 5-HT4 Receptor Agonist RS67333. Int. J. Neuropsychopharm. 2012, 15, 631–643.
- Ishii, T.; Kinoshita, K.-i.; Muroi, Y. Serotonin 5-HT4 Receptor Agonists Improve Facilitation of Contextual Fear Extinction in An MPTP-Induced Mouse Model of Parkinson’s Disease. IJMS 2019, 20, 5340.
- Bockaert, J.; Claeysen, S.; Compan, V.; Dumuis, A. 5-HT4 Receptors, a Place in the Sun: Act Two. Curr. Opin. Pharmacol. 2011, 11, 87–93.
- Hagena, H.; Manahan-Vaughan, D. The Serotonergic 5-HT4 Receptor: A Unique Modulator of Hippocampal Synaptic Information Processing and Cognition. Neurobiol. Learn. Memory 2017, 138, 145–153.
- Teixeira, C.M.; Rosen, Z.B.; Suri, D.; Sun, Q.; Hersh, M.; Sargin, D.; Dincheva, I.; Morgan, A.A.; Spivack, S.; Krok, A.C.; et al. Hippocampal 5-HT Input Regulates Memory Formation and Schaffer Collateral Excitation. Neuron 2018, 98, 992–1004.e4.
- Segu, L.; Lecomte, M.-J.; Wolff, M.; Santamaria, J.; Hen, R.; Dumuis, A.; Berrard, S.; Bockaert, J.; Buhot, M.-C.; Compan, V. Hyperfunction of Muscarinic Receptor Maintains Long-Term Memory in 5-HT4 Receptor Knock-Out Mice. PLoS ONE 2010, 5, e9529.
- Lelong, V.; Dauphin, F.; Boulouard, M. RS 67333 and D-Cycloserine Accelerate Learning Acquisition in the Rat. Neuropharmacology 2001, 41, 517–522.
- Lamirault, L.; Simon, H. Enhancement of Place and Object Recognition Memory in Young Adult and Old Rats by RS 67333, a Partial Agonist of 5-HT4 Receptors. Neuropharmacology 2001, 41, 844–853.
- Freret, T.; Bouet, V.; Quiedeville, A.; Nee, G.; Dallemagne, P.; Rochais, C.; Boulouard, M. Synergistic Effect of Acetylcholinesterase Inhibition (Donepezil) and 5-HT4 Receptor Activation (RS67333) on Object Recognition in Mice. Behav. Brain Res. 2012, 230, 304–308.
- Levallet, G.; Hotte, M.; Boulouard, M.; Dauphin, F. Increased Particulate Phosphodiesterase 4 in the Prefrontal Cortex Supports 5-HT4 Receptor-Induced Improvement of Object Recognition Memory in the Rat. Psychopharmacology 2009, 202, 125–139.
- Marchetti-Gauthier, E.; Roman, F.S.; Dumuis, A.; Bockaert, J.; Soumireu-Mourat, B. BIMU1 Increases Associative Memory in Rats by Activating 5-HT4 Receptors. Neuropharmacology 1997, 36, 697–706.
- Galeotti, N.; Ghelardini, C.; Bartolini, A. Role of 5-HT4 Receptors in the Mouse Passive Avoidance Test. J. Pharmacol. Exp. Ther. 1998, 286, 1115–1121.
- Matsumoto, M.; Togashi, H.; Mori, K.; Ueno, K.; Ohashi, S.; Kojima, T.; Yoshioka, M. Evidence for Involvement of Central 5-HT(4) Receptors in Cholinergic Function Associated with Cognitive Processes: Behavioral, Electrophysiological, and Neurochemical Studies. J. Pharmacol. Exp. Ther. 2001, 296, 676–682.
- Moser, P.C.; Bergis, O.E.; Jegham, S.; Lochead, A.; Duconseille, E.; Terranova, J.-P.; Caille, D.; Berque-Bestel, I.; Lezoualc’h, F.; Fischmeister, R.; et al. SL65.0155, a Novel 5-Hydroxytryptamine(4) Receptor Partial Agonist with Potent Cognition-Enhancing Properties. J. Pharmacol. Exp. Ther. 2002, 302, 731–741.
- Baranger, K.; Giannoni, P.; Girard, S.D.; Girot, S.; Gaven, F.; Stephan, D.; Migliorati, M.; Khrestchatisky, M.; Bockaert, J.; Marchetti-Gauthier, E.; et al. Chronic Treatments with a 5-HT4 Receptor Agonist Decrease Amyloid Pathology in the Entorhinal Cortex and Learning and Memory Deficits in the 5xFAD Mouse Model of Alzheimer’s Disease. Neuropharmacology 2017, 126, 128–141.
- Giannoni, P.; Gaven, F.; de Bundel, D.; Baranger, K.; Marchetti-Gauthier, E.; Roman, F.S.; Valjent, E.; Marin, P.; Bockaert, J.; Rivera, S.; et al. Early Administration of RS 67333, a Specific 5-HT4 Receptor Agonist, Prevents Amyloidogenesis and Behavioral Deficits in the 5XFAD Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2013, 5, 96.
- Eydipour, Z.; Nasehi, M.; Vaseghi, S.; Jamaldini, S.H.; Zarrindast, M.-R. The Role of 5-HT4 Serotonin Receptors in the CA1 Hippocampal Region on Memory Acquisition Impairment Induced by Total (TSD) and REM Sleep Deprivation (RSD). Physiol. Behav. 2020, 215, 112788.
- Murphy, S.E.; de Cates, A.N.; Gillespie, A.L.; Godlewska, B.R.; Scaife, J.C.; Wright, L.C.; Cowen, P.J.; Harmer, C.J. Translating the Promise of 5HT 4 Receptor Agonists for the Treatment of Depression. Psychol. Med. 2021, 51, 1111–1120.
- Cachard-chastel, M.; Devers, S.; Sicsic, S.; Langlois, M.; Lezoualch, F.; Gardier, A.; Belzung, C. Prucalopride and Donepezil Act Synergistically to Reverse Scopolamine-Induced Memory Deficit in C57Bl/6j Mice. Behav. Brain Res. 2008, 187, 455–461.
- Lecoutey, C.; Hedou, D.; Freret, T.; Giannoni, P.; Gaven, F.; Since, M.; Bouet, V.; Ballandonne, C.; Corvaisier, S.; Malzert Freon, A.; et al. Design of Donecopride, a Dual Serotonin Subtype 4 Receptor Agonist/Acetylcholinesterase Inhibitor with Potential Interest for Alzheimer’s Disease Treatment. Proc. Natl. Acad. Sci. USA 2014, 111, E3825–E3830.
- Jansen, C.U.; Qvortrup, K.M. Small Molecule Drugs for Treatment of Alzheimer’s Diseases Developed on the Basis of Mechanistic Understanding of the Serotonin Receptors 4 and 6; IntechOpen: London, UK, 2021.
- Rebholz, H.; Friedman, E.; Castello, J. Alterations of Expression of the Serotonin 5-HT4 Receptor in Brain Disorders. IJMS 2018, 19, 3581.
- Bonaventure, P.; Hall, H.; Gommeren, W.; Cras, P.; Langlois, X.; Jurzak, M.; Leysen, J.E. Mapping of Serotonin 5-HT(4) Receptor MRNA and Ligand Binding Sites in the Post-Mortem Human Brain. Synapse 2000, 36, 35–46.
- Marner, L.; Gillings, N.; Madsen, K.; Erritzoe, D.; Baaré, W.F.C.; Svarer, C.; Hasselbalch, S.G.; Knudsen, G.M. Brain Imaging of Serotonin 4 Receptors in Humans with SB207145-PET. NeuroImage 2010, 50, 855–861.
- Beliveau, V.; Ganz, M.; Feng, L.; Ozenne, B.; Højgaard, L.; Fisher, P.M.; Svarer, C.; Greve, D.N.; Knudsen, G.M. A High-Resolution In Vivo Atlas of the Human Brain’s Serotonin System. J. Neurosci. 2017, 37, 120–128.
- Vilaró, M.T.; Cortés, R.; Mengod, G. Serotonin 5-HT4 Receptors and Their MRNAs in Rat and Guinea Pig Brain: Distribution and Effects of Neurotoxic Lesions: 5-HT4 Receptors in Rat and Guinea Pig Brain. J. Comp. Neurol. 2005, 484, 418–439.
- Tanaka, K.F.; Samuels, B.A.; Hen, R. Serotonin Receptor Expression along the Dorsal–Ventral Axis of Mouse Hippocampus. Phil. Trans. R. Soc. B 2012, 367, 2395–2401.
- Haahr, M.E.; Fisher, P.; Holst, K.; Madsen, K.; Jensen, C.G.; Marner, L.; Lehel, S.; Baaré, W.; Knudsen, G.; Hasselbalch, S. The 5-HT4 Receptor Levels in Hippocampus Correlates Inversely with Memory Test Performance in Humans: The 5-HT4 Receptor and Memory Functions. Hum. Brain Mapp. 2013, 34, 3066–3074.
- Madsen, K.; Neumann, W.-J.; Holst, K.; Marner, L.; Haahr, M.T.; Lehel, S.; Knudsen, G.M.; Hasselbalch, S.G. Cerebral Serotonin 4 Receptors and Amyloid-β in Early Alzheimer’s Disease. JAD 2011, 26, 457–466.
- Meneses, A. Serotonin, Neural Markers, and Memory. Front. Pharmacol. 2015, 6, 143.
- Reynolds, G.P.; Mason, S.L.; Meldrum, A.; Keczer, S.; Parties, H.; Eglen, R.M.; Wong, E.H.F. 5-Hydroxytryptamine (5-HT)4 Receptors in Post Mortem Human Brain Tissue: Distribution, Pharmacology and Effects of Neurodegenerative Diseases. Br. J. Pharmacol. 1995, 114, 993–998.
- Vidal, R.; Valdizán, E.M.; Mostany, R.; Pazos, A.; Castro, E. Long-Term Treatment with Fluoxetine Induces Desensitization of 5-HT4 Receptor-Dependent Signalling and Functionality in Rat Brain. J. Neurochem. 2009, 110, 1120–1127.
- Licht, C.L.; Knudsen, G.M.; Sharp, T. Effects of the 5-HT4 Receptor Agonist RS67333 and Paroxetine on Hippocampal Extracellular 5-HT Levels. Neurosci. Lett. 2010, 476, 58–61.
- Ge, J.; Barnes, N.M. 5-HT4 Receptor-Mediated Modulation of 5-HT Release in the Rat Hippocampus in Vivo. Br. J. Pharmacol. 1996, 117, 1475–1480.
- Conductier, G.; Dusticier, N.; Lucas, G.; Côté, F.; Debonnel, G.; Daszuta, A.; Dumuis, A.; Nieoullon, A.; Hen, R.; Bockaert, J.; et al. Adaptive Changes in Serotonin Neurons of the Raphe Nuclei in 5-HT4 Receptor Knock-out Mouse. Eur. J. Neurosci. 2006, 24, 1053–1062.
- Halliday, G.M.; McCann, H.L.; Pamphlett, R.; Brooks, W.S.; Creasey, H.; McCusker, E.; Cotton, R.G.H.; Broe, G.A.; Harper, C.G. Brain Stem Serotonin-Synthesizing Neurons in Alzheimer’s Disease: A Clinicopathological Correlation. Acta Neuropathol. 1992, 84, 638–650.
- Ebinger, G.; Bruyland, M.; Martin, J.J.; Herregodts, P.; Cras, P.; Michotte, Y.; Gommé, L. Distribution of Biogenic Amines and Their Catabolites in Brains from Patients with Alzheimer’s Disease. J. Neurol. Sci. 1987, 77, 267–283.
- Bowen, D.M.; Allen, S.J.; Benton, J.S.; Goodhardt, M.J.; Haan, E.A.; Palmer, A.M.; Sims, N.R.; Smith, C.C.T.; Spillane, J.A.; Esiri, M.M.; et al. Biochemical Assessment of Serotonergic and Cholinergic Dysfunction and Cerebral Atrophy in Alzheimer’s Disease. J. Neurochem. 1983, 41, 266–272.
- Buhot, M.-C.; Martin, S.; Segu, L. Role of Serotonin in Memory Impairment. Ann. Med. 2000, 32, 210–221.
- Small, S.A.; Schobel, S.A.; Buxton, R.B.; Witter, M.P.; Barnes, C.A. A Pathophysiological Framework of Hippocampal Dysfunction in Ageing and Disease. Nat. Rev. Neurosci. 2011, 12, 585–601.
- Yassa, M.A.; Mattfeld, A.T.; Stark, S.M.; Stark, C.E.L. Age-Related Memory Deficits Linked to Circuit-Specific Disruptions in the Hippocampus. Proc. Natl. Acad. Sci. USA 2011, 108, 8873–8878.
- Jack, C.R.; Petersen, R.C.; Xu, Y.; O’Brien, P.C.; Smith, G.E.; Ivnik, R.J.; Boeve, B.F.; Tangalos, E.G.; Kokmen, E. Rates of Hippocampal Atrophy Correlate with Change in Clinical Status in Aging and AD. Neurology 2000, 55, 484–489.
- Sasabayashi, D.; Yoshimura, R.; Takahashi, T.; Takayanagi, Y.; Nishiyama, S.; Higuchi, Y.; Mizukami, Y.; Furuichi, A.; Kido, M.; Nakamura, M.; et al. Reduced Hippocampal Subfield Volume in Schizophrenia and Clinical High-Risk State for Psychosis. Front. Psychiatry 2021, 12, 642048.
- Xu, R.; Hu, X.; Jiang, X.; Zhang, Y.; Wang, J.; Zeng, X. Longitudinal Volume Changes of Hippocampal Subfields and Cognitive Decline in Parkinson’s Disease. Quant. Imaging Med. Surg. 2020, 10, 220–232.
- Santos, M.A.O.; Bezerra, L.S.; Carvalho, A.R.M.R.; Brainer-Lima, A.M. Global Hippocampal Atrophy in Major Depressive Disorder: A Meta-Analysis of Magnetic Resonance Imaging Studies. Trends Psychiatry Psychother. 2018, 40, 369–378.
- Ruan, L.; Lau, B.W.-M.; Wang, J.; Huang, L.; ZhuGe, Q.; Wang, B.; Jin, K.; So, K.-F. Neurogenesis in Neurological and Psychiatric Diseases and Brain Injury: From Bench to Bedside. Progress Neurobiol. 2014, 115, 116–137.
- Cachard-Chastel, M.; Lezoualc’h, F.; Dewachter, I.; Deloménie, C.; Croes, S.; Devijver, H.; Langlois, M.; Van Leuven, F.; Sicsic, S.; Gardier, A.M. 5-HT4 Receptor Agonists Increase SAPPα Levels in the Cortex and Hippocampus of Male C57BL/6j Mice: 5-HT4 Receptors and SAPPα in Vivo. Br. J. Pharmacol. 2007, 150, 883–892.
- Mohler, E.G.; Shacham, S.; Noiman, S.; Lezoualc’h, F.; Robert, S.; Gastineau, M.; Rutkowski, J.; Marantz, Y.; Dumuis, A.; Bockaert, J.; et al. VRX-03011, a Novel 5-HT4 Agonist, Enhances Memory and Hippocampal Acetylcholine Efflux. Neuropharmacology 2007, 53, 563–573.
- Lezoualc’h, F.; Robert, S.J. The Serotonin 5-HT4 Receptor and the Amyloid Precursor Protein Processing. Exp. Gerontol. 2003, 38, 159–166.
- Robert, S.J.; Zugaza, J.L.; Fischmeister, R.; Gardier, A.M.; Lezoualc’h, F. The Human Serotonin 5-HT4 Receptor Regulates Secretion of Non-Amyloidogenic Precursor Protein. J. Biol. Chem. 2001, 276, 44881–44888.
- Maillet, M.; Robert, S.J.; Cacquevel, M.; Gastineau, M.; Vivien, D.; Bertoglio, J.; Zugaza, J.L.; Fischmeister, R.; Lezoualc’h, F. Crosstalk between Rap1 and Rac Regulates Secretion of SAPPalpha. Nat. Cell Biol. 2003, 5, 633–639.
- Cochet, M.; Donneger, R.; Cassier, E.; Gaven, F.; Lichtenthaler, S.F.; Marin, P.; Bockaert, J.; Dumuis, A.; Claeysen, S. 5-HT4 Receptors Constitutively Promote the Non-Amyloidogenic Pathway of APP Cleavage and Interact with ADAM10. ACS Chem. Neurosci. 2013, 4, 130–140.
- Hashimoto, G.; Sakurai, M.; Teich, A.F.; Saeed, F.; Aziz, F.; Arancio, O. 5-HT₄ Receptor Stimulation Leads to Soluble AβPPα Production through MMP-9 Upregulation. J. Alzheimer’s Dis. 2012, 32, 437–445.
- Tesseur, I.; Pimenova, A.A.; Lo, A.C.; Ciesielska, M.; Lichtenthaler, S.F.; De Maeyer, J.H.; Schuurkes, J.A.J.; D’Hooge, R.; De Strooper, B. Chronic 5-HT4 Receptor Activation Decreases Aβ Production and Deposition in HAPP/PS1 Mice. Neurobiol. Aging 2013, 34, 1779–1789.
- Cho, S.; Hu, Y. Activation of 5-HT4 Receptors Inhibits Secretion of β-Amyloid Peptides and Increases Neuronal Survival. Exp. Neurol. 2007, 203, 274–278.
- Koffie, R.M.; Hyman, B.T.; Spires-Jones, T.L. Alzheimer’s Disease: Synapses Gone Cold. Mol. Neurodegeneration 2011, 6, 63.
- Schill, Y.; Bijata, M.; Kopach, O.; Cherkas, V.; Abdel-Galil, D.; Böhm, K.; Schwab, M.H.; Matsuda, M.; Compan, V.; Basu, S.; et al. Serotonin 5-HT4 Receptor Boosts Functional Maturation of Dendritic Spines via RhoA-Dependent Control of F-Actin. Commun. Biol. 2020, 3, 76.
- Kozono, N.; Ohtani, A.; Shiga, T. Roles of the Serotonin 5-HT4 Receptor in Dendrite Formation of the Rat Hippocampal Neurons in Vitro. Brain Res. 2017, 1655, 114–121.
- Preman, P.; Alfonso-Triguero, M.; Alberdi, E.; Verkhratsky, A.; Arranz, A.M. Astrocytes in Alzheimer’s Disease: Pathological Significance and Molecular Pathways. Cells 2021, 10, 540.
- Saura, C.A.; Valero, J. The Role of CREB Signaling in Alzheimer’s Disease and Other Cognitive Disorders. Rev. Neurosci. 2011, 22, 153–169.
- Susannah E. Murphy; Lucy C. Wright; Michael Browning; Philip J. Cowen; Catherine J. Harmer; A role for 5-HT4 receptors in human learning and memory. Psychological Medicine 2019, 50, 2722-2730, 10.1017/s0033291719002836.
- Angharad N. de Cates; Lucy C. Wright; Marieke A. G. Martens; Daisy Gibson; Cagdas Türkmen; Nicola Filippini; Philip J. Cowen; Catherine J. Harmer; Susannah E. Murphy; Déjà-vu? Neural and behavioural effects of the 5-HT4 receptor agonist, prucalopride, in a hippocampal-dependent memory task. Translational Psychiatry 2021, 11, 1-9, 10.1038/s41398-021-01568-4.
