Microbial biofilms occur naturally in many environmental niches and can be a significant reservoir of infectious microbes in zoonotically transmitted diseases such as that caused by Campylobacter jejuni, the leading cause of acute human bacterial gastroenteritis world-wide. The greatest challenge in reducing the disease caused by this organism is reducing transmission of C. jejuni to humans from poultry via the food chain. Biofilms enhance the stress tolerance and antimicrobial resistance of the microorganisms they harbor and are considered to play a crucial role for Campylobacter spp. survival and transmission to humans. Unconventional approaches to control biofilms and to improve the efficacy of currently used antibiotics are urgently needed. This review summarizes the use plant- and microorganism-derived antimicrobial and antibiofilm compounds such as essential oils, antimicrobial peptides (AMPs), polyphenolic extracts, algae extracts, probiotic-derived factors, d-amino acids (DAs) and glycolipid biosurfactants with potential to control biofilms formed by Campylobacter, and the suggested mechanisms of their action. Further investigation and use of such natural compounds could improve preventative and remedial strategies aimed to limit the transmission of campylobacters and other human pathogens via the food chain.
- Campylobacter
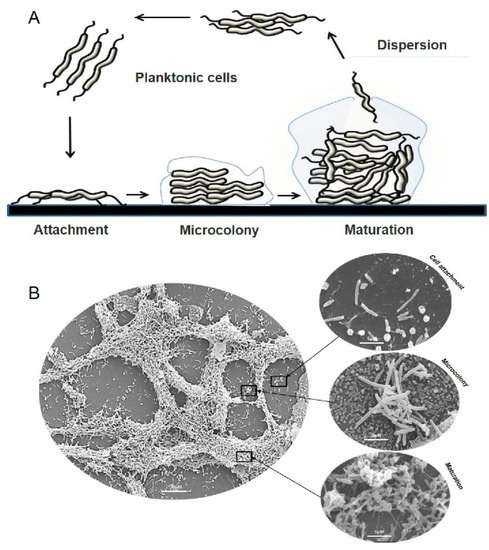
1. Campylobacter spp. Biofilm Formation and Regulation
2. Natural Antibiofilm Compounds
| Compounds | Mechanism of Action | Strains | MIC * | References | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant-derived compounds | ||||||||||||||||||||
| Essential oils (EOs) |
|
| C. jejuni | NCTC 11168 | C. coli | | C. jejuni | S-8 | C. jejuni | NCTC 81-176 | C. jejuni | RC039 | 1.76 mg/L (75.64 mM) | [70,71] | [47][48] | |||||
| 0.05–0.4 mg/mL | [72] | [49] | |||||||||||||||||
| 2.69 mg/L (60.9 mM) | [73] | [50] | |||||||||||||||||
| 31.25 mg/L (66.56 mM) | [74] | [51] | |||||||||||||||||
| 1 mg/mL | [75] | [52] | |||||||||||||||||
| 1 mg/mL | [74,76] | [51][53] | |||||||||||||||||
| 125 mg/L | [77] | [54] | |||||||||||||||||
| Plant extracts |
|
| C. jejuni | NCTC 11168 | C. jejuni | S-8 | C. jejuni | F38011 | C. jejuni | 180ip | C. jejuni | 238ip | C. coli | 60 mg/L | [78] | [55] | ||||
| 225 µg/mL | [79] | [56] | |||||||||||||||||
| 64–1024 µg/mL | [80] | [57] | |||||||||||||||||
| 50 μg/mL | [81,82] | [58][59] | |||||||||||||||||
| 0.15–0.3 mg/L | [83] | [60] | |||||||||||||||||
| 0.1–0.2 mg/mL | [84] | [61] | |||||||||||||||||
| 0.04 mg/mL | [85] | [62] | |||||||||||||||||
| Antimicrobial peptides (AMPs) | Puroindoline A (PinA) |
| C. jejuni | 81-176 | 512 μg/mL | [56,86,87] | [33][63][64] | |||||||||||||
| Microorganism-derived compounds | ||||||||||||||||||||
| Algae extracts | Delisea pulchra | extract |
| C. jejuni | NCTC 11168 | 230 µg/mL | [88] | [65] | ||||||||||||
| d | -amino acids (DAs) |
|
| C. jejuni | NCTC 11168 | 5–100 mM | [47] | [24] | ||||||||||||
| Probiotic-derived factors |
|
| C. jejuni | C. coli | 0.025–32 µg/mL 1.5–5.8 μM |
[89] [90] | [66] [67] |
|||||||||||||
| Glycolipid Biosurfactant | Sophorolipid |
| C. jejuni | subsp. | jejuni | 33560 | 0.003% | [88] | [65] | |||||||||||
2.1. Plant-Derived Compounds
References
- Joshua, G.P.; Guthrie-Irons, C.; Karlyshev, A.; Wren, B. Biofilm formation in Campylobacter jejuni. Microbiology 2006, 152, 387–396.
- Brown, H.L.; Reuter, M.; Salt, L.J.; Cross, K.L.; Betts, R.P.; van Vliet, A.H. Chicken juice enhances surface attachment and biofilm formation of Campylobacter jejuni. Appl. Environ. Microbiol. 2014, 80, 7053–7060.
- Bronowski, C.; James, C.E.; Winstanley, C. Role of environmental survival in transmission of Campylobacter jejuni. FEMS Microbiol. Lett. 2014, 356, 8–19.
- Reuter, M.; Mallett, A.; Pearson, B.M.; van Vliet, A.H. Biofilm formation by Campylobacter jejuni is increased under aerobic conditions. Appl. Environ. Microbiol. 2010, 76, 2122–2128.
- Iovine, N.M. Resistance mechanisms in Campylobacter jejuni. Virulence 2013, 4, 230–240.
- Smith, J.L.; Fratamico, P.M. Fluoroquinolone resistance in Campylobacter. J. Food Prot. 2010, 73, 1141–1152.
- Golz, J.C.; Stingl, K. Natural competence and horizontal gene transfer in Campylobacter. In Fighting Campylobacter Infections: Towards a One Health Approach; Springer Nature: Basingstoke, UK, 2021; pp. 265–292.
- Vegge, C.S.; Brøndsted, L.; Ligowska-Marzęta, M.; Ingmer, H. Natural transformation of Campylobacter jejuni occurs beyond limits of growth. PLoS ONE 2012, 7, e45467.
- Han, J. Molecular Mechanisms Involved in the Emergence and Fitness of Fluoroquinolone-Resistant Campylobacter jejuni; Iowa State University: Ames, IA, USA, 2009.
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed; WHO: Geneva, Switzerland, 2017.
- Silva, J.; Leite, D.; Fernandes, M.; Mena, C.; Gibbs, P.A.; Teixeira, P. Campylobacter spp. as a foodborne pathogen: A review. Front. Microbiol. 2011, 2, 200.
- Whitehouse, C.A.; Zhao, S.; Tate, H. Antimicrobial resistance in Campylobacter species: Mechanisms and genomic epidemiology. Adv. Appl. Microbiol. 2018, 103, 1–47.
- Ica, T.; Caner, V.; Istanbullu, O.; Nguyen, H.D.; Ahmed, B.; Call, D.R.; Beyenal, H. Characterization of mono- and mixed-culture Campylobacter jejuni biofilms. Appl. Environ. Microbiol. 2012, 78, 1033–1038.
- Li, J.; Feng, J.; Ma, L.; de la Fuente Núñez, C.; Gölz, G.; Lu, X. Effects of meat juice on biofilm formation of Campylobacter and Salmonella. Int. J. Food Microbiol. 2017, 253, 20–28.
- Scheik, L.K.; Volcan Maia, D.S.; Würfel, S.D.F.R.; Ramires, T.; Kleinubing, N.R.; Haubert, L.; Lopes, G.V.; da Silva, W.P. Biofilm-forming ability of poultry Campylobacter jejuni strains in the presence and absence of Pseudomonas aeruginosa. Can. J. Microbiol. 2021, 67, 301–309.
- World Health Organization. The Global View of Campylobacteriosis: Report of an Expert Consultation, Utrecht, Netherlands, 9–11 July 2012; WHO: Geneva, Switzerland, 2013.
- Mah, T.-F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072.
- Brown, H.L.; Reuter, M.; Hanman, K.; Betts, R.P.; van Vliet, A.H. Prevention of Biofilm Formation and Removal of Existing Biofilms by Extracellular DNases of Campylobacter jejuni. PLoS ONE 2015, 10, e0121680.
- Svensson, S.L.; Pryjma, M.; Gaynor, E.C. Flagella-mediated adhesion and extracellular DNA release contribute to biofilm formation and stress tolerance of Campylobacter jejuni. PLoS ONE 2014, 9, e106063.
- Turonova, H.; Briandet, R.; Rodrigues, R.; Hernould, M.; Hayek, N.; Stintzi, A.; Pazlarova, J.; Tresse, O. Biofilm spatial organization by the emerging pathogen Campylobacter jejuni: Comparison between NCTC 11168 and 81-176 strains under microaerobic and oxygen-enriched conditions. Front. Microbiol. 2015, 6, 709.
- Gaasbeek, E.J.; Wagenaar, J.A.; Guilhabert, M.R.; Wösten, M.M.; van Putten, J.P.; van der Graaf-van Bloois, L.; Parker, C.T.; van der Wal, F.J. A DNase encoded by integrated element CJIE1 inhibits natural transformation of Campylobacter jejuni. J. Bacteriol. 2009, 191, 2296–2306.
- Van Houdt, R.; Michiels, C.W. Role of bacterial cell surface structures in Escherichia coli biofilm formation. Res. Microbiol. 2005, 156, 626–633.
- Turonova, H.; Neu, T.R.; Ulbrich, P.; Pazlarova, J.; Tresse, O. The biofilm matrix of Campylobacter jejuni determined by fluorescence lectin-binding analysis. Biofouling 2016, 32, 597–608.
- Elgamoudi, B.A.; Taha, T.; Korolik, V. Inhibition of Campylobacter jejuni biofilm formation by d-amino acids. Antibiotics 2020, 9, 836.
- Palmer, J.; Flint, S.; Brooks, J. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol. 2007, 34, 577–588.
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Micro. 2010, 8, 623–633.
- Melo, R.T.; Mendonça, E.P.; Monteiro, G.P.; Siqueira, M.C.; Pereira, C.B.; Peres, P.A.; Fernandez, H.; Rossi, D.A. Intrinsic and extrinsic aspects on Campylobacter jejuni biofilms. Front. Microbiol. 2017, 8, 1332.
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic Era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306.
- Das, T.; Manefield, M. Pyocyanin promotes extracellular DNA release in Pseudomonas aeruginosa. PLoS ONE 2012, 7, e46718.
- Kaplan, J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218.
- Ma, L.; Conover, M.; Lu, H.; Parsek, M.R.; Bayles, K.; Wozniak, D.J. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog 2009, 5, e1000354.
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355.
- Boles, B.R.; Horswill, A.R. Staphylococcal biofilm disassembly. Trends Microbiol. 2011, 19, 449–455.
- Rendueles, O.; Ghigo, J.-M. Multi-species biofilms: How to avoid unfriendly neighbors. FEMS Microbiol. Rev. 2012, 36, 972–989.
- Tram, G.; Day, C.J.; Korolik, V. Bridging the gap: A role for Campylobacter jejuni biofilms. Microorganisms 2020, 8, 452.
- Whelan, M.V.; Simpson, J.C.; Cróinín, T.Ó. A novel high-content screening approach for the elucidation of Campylobacter jejuni biofilm composition and integrity. BMC Microbiol. 2021, 21, 2.
- Püning, C.; Su, Y.; Lu, X.; Gölz, G. Molecular mechanisms of Campylobacter biofilm formation and quorum sensing. In Fighting Campylobacter Infections: Towards a One Health Approach; Springer: Berlin/Heidelberg, Germany, 2021; pp. 293–319.
- Lim, E.S.; Kim, J.-S. Role of eptC in biofilm formation by Campylobacter jejuni NCTC11168 on polystyrene and glass surfaces. J. Microbiol. Biotechnol. 2017, 27, 1609–1616.
- Burnham, P.M.; Hendrixson, D.R. Campylobacter jejuni: Collective components promoting a successful enteric lifestyle. Nat. Rev. Microbiol. 2018, 16, 551–565.
- Guerry, P.; Poly, F.; Riddle, M.; Maue, A.C.; Chen, Y.H.; Monteiro, M.A. Campylobacter polysaccharide capsules: Virulence and vaccines. Front. Cell. Infect. Microbiol. 2012, 2, 7.
- Teren, M.; Michova, H.T.; Vondrakova, L.; Demnerova, K. Molecules autoinducer 2 and cjA and their impact on gene expression in Campylobacter jejuni. J. Mol. Microbiol. Biotechnol. 2018, 28, 207–215.
- Tereň, M.; Shagieva, E.; Vondrakova, L.; Viktorova, J.; Svarcova, V.; Demnerova, K.; Michova, H.T. Mutagenic strategies against luxS gene affect the early stage of biofilm formation of Campylobacter jejuni. J. Appl. Genet. 2021.
- Tischler, A.D.; Camilli, A. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol. Microbiol. 2004, 53, 857–869.
- Balta, I.; Linton, M.; Pinkerton, L.; Kelly, C.; Stef, L.; Pet, I.; Stef, D.; Criste, A.; Gundogdu, O.; Corcionivoschi, N. The effect of natural antimicrobials against Campylobacter spp. and its similarities to Salmonella spp, Listeria spp., Escherichia coli, Vibrio spp., Clostridium spp. and Staphylococcus spp. Food Control 2020, 121, 107745.
- Kalogianni, A.I.; Lazou, T.; Bossis, I.; Gelasakis, A.I. Natural phenolic compounds for the control of oxidation, bacterial spoilage, and foodborne pathogens in meat. Foods 2020, 9, 794.
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural anti-biofilm agents: Strategies to control biofilm-forming pathogens. Front. Microbiol. 2020, 11, 2640.
- Wagle, B.R.; Arsi, K.; Shrestha, S.; Upadhyay, A.; Upadhyaya, I.; Bhargava, K.; Donoghue, A.; Donoghue, D.J. Eugenol as an antimicrobial wash treatment reduces Campylobacter jejuni in postharvest poultry. J. Food Saf. 2019, 39, e12704.
- Upadhyaya, I.; Upadhyay, A.; Arsi, K.; Liyanage, R.; Donoghue, A.; Rath, N.; Donoghue, D. Plant-derived antimicrobial eugenol modulates Campylobacter jejun proteome and virulence critical for colonization in chickens. Poult. Sci. 2017, 96, 537.
- Wagle, B.R.; Upadhyay, A.; Upadhyaya, I.; Shrestha, S.; Arsi, K.; Liyanage, R.; Venkitanarayanan, K.; Donoghue, D.J.; Donoghue, A.M. Trans-cinnamaldehyde, eugenol and carvacrol reduce Campylobacter jejuni biofilms and modulate expression of select genes and proteins. Front. Microbiol. 2019, 10, 1837.
- Yu, H.H.; Song, Y.J.; Yu, H.S.; Lee, N.K.; Paik, H.D. Investigating the antimicrobial and antibiofilm effects of cinnamaldehyde against Campylobacter spp. using cell surface characteristics. J. Food Sci. 2020, 85, 157–164.
- Šimunović, K.; Ramić, D.; Xu, C.; Smole Možina, S. Modulation of Campylobacter jejuni motility, adhesion to polystyrene surfaces, and invasion of INT407 cells by quorum-sensing inhibition. Microorganisms 2020, 8, 104.
- Gahamanyi, N.; Song, D.-G.; Cha, K.H.; Yoon, K.-Y.; Mboera, L.E.; Matee, M.I.; Mutangana, D.; Amachawadi, R.G.; Komba, E.V.; Pan, C.-H. Susceptibility of Campylobacter strains to selected natural products and frontline antibiotics. Antibiotics 2020, 9, 790.
- Klančnik, A.; Šimunović, K.; Sterniša, M.; Ramić, D.; Možina, S.S.; Bucar, F. Anti-adhesion activity of phytochemicals to prevent Campylobacter jejuni biofilm formation on abiotic surfaces. Phytochem. Rev. 2021, 20, 55–84.
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; LD Jayaweera, S.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N. Therapeutic potential of α-and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738.
- Silván, J.M.; Mingo, E.; Hidalgo, M.; de Pascual-Teresa, S.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Antibacterial activity of a grape seed extract and its fractions against Campylobacter spp. Food Control 2013, 29, 25–31.
- Castillo, S.; Heredia, N.; Arechiga-Carvajal, E.; García, S. Citrus extracts as inhibitors of quorum sensing, biofilm formation and motility of Campylobacter jejuni. Food Biotechnol. 2014, 28, 106–122.
- Bezek, K.; Kurinčič, M.; Knauder, E.; Klančnik, A.; Raspor, P.; Bucar, F.; Smole Možina, S. Attenuation of adhesion, biofilm formation and quorum sensing of Campylobacter jejuni by Euodia ruticarpa. Phytother. Res. 2016, 30, 1527–1532.
- Wagle, B.R.; Donoghue, A.M.; Jesudhasan, P.R. Select phytochemicals reduce Campylobacter jejuni in postharvest poultry and modulate the virulence attributes of C. jejuni. Front. Microbiol. 2021, 2270.
- Lu, X.; Samuelson, D.R.; Rasco, B.A.; Konkel, M.E. Antimicrobial effect of diallyl sulphide on Campylobacter jejuni biofilms. J. Antimicrob. Chemother. 2012, 67, 1915–1926.
- Castillo, S.; Heredia, N.; García, S. 2 (5H)-Furanone, epigallocatechin gallate, and a citric-based disinfectant disturb quorum-sensing activity and reduce motility and biofilm formation of Campylobacter jejuni. Folia Microbiol. 2015, 60, 89–95.
- Roila, R.; Ranucci, D.; Valiani, A.; Galarini, R.; Servili, M.; Branciari, R. Antimicrobial and anti-biofilm activity of olive oil by-products against Campylobacter spp. isolated from chicken meat. Acta Sci. Pol. Technol. Aliment. 2019, 18, 43–52.
- Duarte, A.; Alves, A.C.; Ferreira, S.; Silva, F.; Domingues, F.C. Resveratrol inclusion complexes: Antibacterial and anti-biofilm activity against Campylobacter spp. and Arcobacter butzleri. Food Res. Int. 2015, 77, 244–250.
- Romeo, T. When the party is over: A signal for dispersal of Pseudomonas aeruginosa biofilms. J. Bacteriol. 2006, 188, 7325–7327.
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2012, 10, 39–50.
- Silveira, V.A.I.; Nishio, E.K.; Freitas, C.A.; Amador, I.R.; Kobayashi, R.K.; Caretta, T.; Macedo, F.; Celligoi, M.A.P. Production and antimicrobial activity of sophorolipid against Clostridium perfringens and Campylobacter jejuni and their additive interaction with lactic acid. Biocatal. Agric. Biotechnol. 2019, 21, 101287.
- Asare, P.T.; Zurfluh, K.; Greppi, A.; Lynch, D.; Schwab, C.; Stephan, R.; Lacroix, C. Reuterin demonstrates potent antimicrobial activity against a broad panel of human and poultry meat Campylobacter spp. isolates. Microorganisms 2020, 8, 78.
- Svetoch, E.A.; Eruslanov, B.V.; Perelygin, V.V.; Mitsevich, E.V.; Mitsevich, I.P.; Borzenkov, V.N.; Levchuk, V.P.; Svetoch, O.E.; Kovalev, Y.N.; Stepanshin, Y.G. Diverse antimicrobial killing by Enterococcus faecium E 50–52 bacteriocin. J. Agric. Food Chem. 2008, 56, 1942–1948.
- Micciche, A.; Rothrock Jr, M.J.; Yang, Y.; Ricke, S.C. Essential oils as an intervention strategy to reduce Campylobacter in poultry production: A review. Front. Microbiol. 2019, 10, 1058.
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 11.
- Abd Rashed, A.; Rathi, D.-N.G.; Ahmad Nasir, N.A.H.; Abd Rahman, A.Z. Antifungal properties of essential oils and their compounds for application in skin fungal infections: Conventional and nonconventional approaches. Molecules 2021, 26, 1093.
- Mucha, W.; Witkowska, D. The applicability of essential oils in different stages of production of animal-based foods. Molecules 2021, 26, 3798.
- Kurekci, C.; Padmanabha, J.; Bishop-Hurley, S.L.; Hassan, E.; Al Jassim, R.A.; McSweeney, C.S. Antimicrobial activity of essential oils and five terpenoid compounds against Campylobacter jejuni in pure and mixed culture experiments. Int. J. Food Microbiol. 2013, 166, 450–457.
- De Oliveira, M.M.M.; Brugnera, D.F.; das Graças Cardoso, M.; Alves, E.; Piccoli, R.H. Disinfectant action of Cymbopogon sp. essential oils in different phases of biofilm formation by Listeria monocytogenes on stainless steel surface. Food Control 2010, 21, 549–553.
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017, 7, 8211.
- Valeriano, C.; De Oliveira, T.L.C.; De Carvalho, S.M.; das Graças Cardoso, M.; Alves, E.; Piccoli, R.H. The sanitizing action of essential oil-based solutions against Salmonella enterica serotype Enteritidis S64 biofilm formation on AISI 304 stainless steel. Food Control 2012, 25, 673–677.
- Zhai, H.; Liu, H.; Wang, S.; Wu, J.; Kluenter, A.-M. Potential of essential oils for poultry and pigs. Anim. Nutr 2018, 4, 179–186.
- Kirkpinar, F.; Ünlü, H.; Serdaroğlu, M.; Turp, G. Effects of dietary oregano and garlic essential oils on carcass characteristics, meat composition, colour, pH and sensory quality of broiler meat. Br. Poult. Sci. 2014, 55, 157–166.
- Witkowska, D.; Sowińska, J. The effectiveness of peppermint and thyme essential oil mist in reducing bacterial contamination in broiler houses. Poult. Sci. 2013, 92, 2834–2843.
- Topa, S.H.; Subramoni, S.; Palombo, E.A.; Kingshott, P.; Rice, S.A.; Blackall, L.L. Cinnamaldehyde disrupts biofilm formation and swarming motility of Pseudomonas aeruginosa. Microbiology 2018, 164, 1087–1097.
- He, Z.; Huang, Z.; Jiang, W.; Zhou, W. Antimicrobial activity of cinnamaldehyde on Streptococcus mutans biofilms. Front. Microbiol. 2019, 10, 2241.
- Trevisan, D.A.C.; Silva, A.F.D.; Negri, M.; Abreu, B.A.D.; Machinski, M.; Patussi, E.V.; Campanerut-Sá, P.A.Z.; Mikcha, J.M.G. Antibacterial and antibiofilm activity of carvacrol against Salmonella enterica serotype Typhimurium. Braz. J. Pharm. Sci. 2018, 54.
- Upadhyay, A.; Upadhyaya, I.; Kollanoor-Johny, A.; Venkitanarayanan, K. Antibiofilm effect of plant derived antimicrobials on Listeria monocytogenes. Food Microbiol. 2013, 36, 79–89.
- Wagle, B.; Donoghue, A.; Shrestha, S.; Upadhyaya, I.; Arsi, K.; Gupta, A.; Liyanage, R.; Rath, N.; Donoghue, D.; Upadhyay, A. Carvacrol attenuates Campylobacter jejuni colonization factors and proteome critical for persistence in the chicken gut. Poult. Sci. 2020, 99, 4566–4577.
- Johny, A.K.; Darre, M.; Donoghue, A.; Donoghue, D.; Venkitanarayanan, K. Antibacterial effect of trans-cinnamaldehyde, eugenol, carvacrol, and thymol on Salmonella Enteritidis and Campylobacter jejuni in chicken cecal contents in vitro. J. Appl. Poult. Res. 2010, 19, 237–244.
- Kelly, C.; Gundogdu, O.; Pircalabioru, G.; Cean, A.; Scates, P.; Linton, M.; Pinkerton, L.; Magowan, E.; Stef, L.; Simiz, E. The in vitro and in vivo effect of carvacrol in preventing Campylobacter infection, colonization and in improving productivity of chicken broilers. Foodborne Pathog. Dis. 2017, 14, 341–349.
- Allaoua, M.; Etienne, P.; Noirot, V.; Carayon, J.L.; Tene, N.; Bonnafé, E.; Treilhou, M. Pharmacokinetic and antimicrobial activity of a new carvacrol-based product against a human pathogen, Campylobacter jejuni. J. Appl. Microbiol. 2018, 125, 1162–1174.
- Mousavi, S.; Schmidt, A.-M.; Escher, U.; Kittler, S.; Kehrenberg, C.; Thunhorst, E.; Bereswill, S.; Heimesaat, M.M. Carvacrol ameliorates acute campylobacteriosis in a clinical murine infection model. Gut Pathog. 2020, 12, 2.
- Szott, V.; Reichelt, B.; Alter, T.; Friese, A.; Roesler, U. In vivo efficacy of carvacrol on Campylobacter jejuni prevalence in broiler chickens during an entire fattening period. Eur. J. Microbiol. Immunol. 2020, 10, 131–138.
- Simunovic, K.; Bucar, F.; Klancnik, A.; Pompei, F.; Paparella, A.; Mozina, S.S. In vitro effect of the common culinary herb winter savory (Satureja montana) against the infamous food pathogen Campylobacter jejuni. Foods 2020, 9, 537.
- Arsi, K.; Donoghue, A.; Venkitanarayanan, K.; Kollanoor-Johny, A.; Fanatico, A.; Blore, P.; Donoghue, D. The efficacy of the natural plant extracts, thymol and carvacrol against Campylobacter colonization in broiler chickens. J. Food Saf. 2014, 34, 321–325.
- Robyn, J.; Rasschaert, G.; Pasmans, F.; Heyndrickx, M. Thermotolerant Campylobacter during broiler rearing: Risk factors and intervention. Compr. Rev. Food Sci. Food Saf. 2015, 14, 81–105.
- Hashemipour, H.; Kermanshahi, H.; Golian, A.; Veldkamp, T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013, 92, 2059–2069.
- Chowdhury, S.; Mandal, G.P.; Patra, A.K. Different essential oils in diets of chickens: Growth performance, nutrient utilisation, nitrogen excretion, carcass traits and chemical composition of meat. Anim. Feed Sci. Technol. 2018, 236, 86–97.
- Santini, C.; Baffoni, L.; Gaggia, F.; Granata, M.; Gasbarri, R.; Di Gioia, D.; Biavati, B. Characterization of probiotic strains: An application as feed additives in poultry against Campylobacter jejuni. Int. J. Food Microbiol. 2010, 141, S98–S108.
- Minami, M.; Kita, M.; Nakaya, T.; Yamamoto, T.; Kuriyama, H.; Imanishi, J. The inhibitory effect of essential oils on herpes simplex virus type-1 replication in vitro. Microbiol. Immunol. 2003, 47, 681–684.
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents—Myth or real alternative? Molecules 2019, 24, 2130.
- Roller, S.; Ernest, N.; Buckle, J. The antimicrobial activity of high-necrodane and other lavender oils on methicillin-sensitive and-resistant Staphylococcus aureus (MSSA and MRSA). J. Altern. Complement. Med. 2009, 15, 275–279.
- Adaszyńska-Skwirzyńska, M.; Dzięcioł, M. Comparison of phenolic acids and flavonoids contents in various cultivars and parts of common lavender (Lavandula angustifolia) derived from Poland. Nat. Prod. Res. 2017, 31, 2575–2580.
- Adaszyńska-Skwirzyńska, M.; Szczerbińska, D. The antimicrobial activity of lavender essential oil (Lavandula angustifolia) and its influence on the production performance of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1020–1025.
- Císarová, M.; Tančinová, D.; Medo, J. Antifungal activity of lemon, eucalyptus, thyme, oregano, sage and lavender essential oils against Aspergillus niger and Aspergillus tubingensis isolated from grapes. Potravinarstvo 2016, 10, 83–88.
- Ramić, D.; Bucar, F.; Kunej, U.; Dogša, I.; Klančnik, A.; Smole Možina, S. Anti-biofilm potential of Lavandula preparations against Campylobacter jejuni. Appl. Environ. Microbiol. 2021, 87, e01099-21.
- Klančnik, A.; Zorko, Š.; Toplak, N.; Kovač, M.; Bucar, F.; Jeršek, B.; Smole Možina, S. Antiadhesion activity of juniper (Juniperus communis L.) preparations against Campylobacter jejuni evaluated with PCR-based methods. Phytother Res. 2018, 32, 542–550.
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70.
- Khalifaev, P.D.; Sharopov, F.S.; Safomuddin, A.; Numonov, S.; Bakri, M.; Habasi, M.; Aisa, H.A.; Setzer, W.N. Chemical composition of the essential oil from the roots of Ferula kuhistanica growing wild in Tajikistan. Nat. Prod. Commun. 2018, 13, 1934578X1801300226.
- Sharopov, F.; Satyal, P.; Wink, M. Composition of the essential oil of Ferula clematidifolia. Chem. Nat. Compd. 2016, 52, 518–519.
- Šimunović, K.; Sahin, O.; Kovač, J.; Shen, Z.; Klančnik, A.; Zhang, Q.; Smole Možina, S. (-)-α-Pinene reduces quorum sensing and Campylobacter jejuni colonization in broiler chickens. PLoS ONE 2020, 15, e0230423.
