Distillery stillage has a high energy potential (13.6 MJ/kg TS, 10.4 MJ/kg COD), which indicates that it can be processed via anaerobic digestion and is a suitable substrate for conversion into energy. Distillery stillage consists of compounds that are easily biodegraded during anaerobic digestion, such as proteins, lipids, and carbohydrates. Among the carbohydrates, the concentration of cellulose can be on the level of 32.2%, hemicelluloses—20.9%, and lignin—3.2% in the distillery stillage obtained from maize.
- biomethane
- bioelectrochemical treatment
- Distillery Stillage
1. Introduction
The management of industrial waste is a principal area of development in the world, as industrial waste contains a wide variety of organic and inorganic pollutants that have a negative impact on the environment. The distillery industry is one of the main sources of environmental pollution, but also one of the key factors contributing to the development of the global economy. Only 5% of the world’s ethanol production comes from chemical synthesis. More than 95% of ethanol is produced from agricultural raw materials. Of these, sugar-based raw materials account for approximately 42% of the ethanol produced, and non-sugar raw materials (mainly starch-based) account for approximately 58% [1]. Ethanol is produced from cereals (mainly rye, corn, triticale, and wheat), root crops (mainly potatoes), molasses, and other agricultural raw materials [2]. The production of alcohol is constantly growing because it is used in many industries, including the chemical, pharmaceutical, cosmetic, beverage, food, and perfume industries. In addition, the European Union program obliges Member States to use biofuels as transport fuels (their share should amount to 14% in 2025 and 19.7% in 2030) [3]. Along with the increase in the demand for alcohol, the amount of byproducts (termed distillery stillage), which may be up to 15 times greater than the amount of alcohol produced, is also increasing [4]. Treatment of distillery byproducts is a priority area for environmental protection because untreated byproducts that are released into the environment increase water pollution, adversely affect aquatic life, and reduce soil alkalinity. The literature indicates that the most promising option for utilization of distillery byproducts is to valorize them as a renewable feedstock for recovery of energy and biobased materials, thus enabling integration of remediation and recovery of resources. In this biorefinery approach, appropriate technologies are required, including methanogenesis, photosynthesis, photofermentation, dark fermentation, and bioelectrogenesis, which are made possible by the versatile metabolisms of biocatalytic micro-organisms.
2. Processing of Distillery Stillage—Biomethane Production
2.1. Bioreactors and Operational Parameters
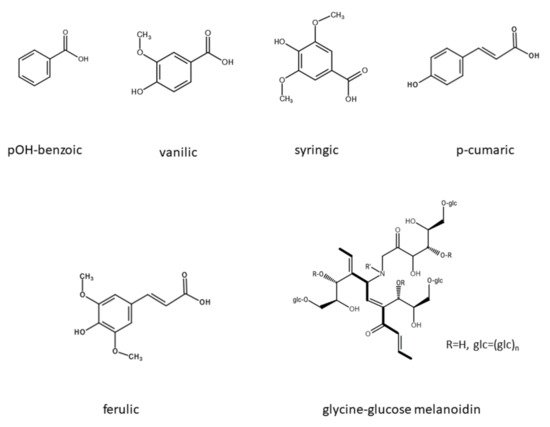
2.2. Effect of Polyphenols and Melanoidin on Biomethane Production
2.3. Pretreatment of Distillery Stillage
2.4. Post-Treatment of Distillery Stillage
References
- Tolmasquim, M.T. Bioenergy for the future. In Proceedings of the Conference on Biofuels: “An Option for a Less Carbon-Intensive Economy”, Rio de Janeiro, Brazil, 4–5 December 2007.
- Krzywonos, M.; Cibis, E.; Miskiewicz, T.; Ryznar-Luty, A. Utilization and biodegradation of starch stillage (distillery wastewater). Electron. J. Biotechnol. 2009, 12, 6–7.
- Krzywonos, M. Forecast for transport biofuels in Poland in 2020–2030. Przemysł Chem. 2015, 1, 168–172.
- Fito, J.; Tefera, N.; van Hulle, S.W.H. Sugarcane biorefineries wastewater: Bioremediation technologies for environmental sustainability. Chem. Biol. Technol. Agric. 2019, 6, 6.
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641.
- Schnürer, A.; Nordberg, Å. Ammonia, a selective agent for methane production by syntrophic acetate oxidation at mesophilic temperature. Water Sci. Technol. 2008, 57, 735–740.
- Procházka, J.; Dolejš, P.; Máca, J.; Dohányos, M. Stability and inhibition of anaerobic processes caused by insufficiency or excess of ammonia nitrogen. Appl. Microbiol. Biotechnol. 2012, 93, 439–447.
- Moestedt, J.; Nordell, E.; Schnürer, A. Comparison of operating strategies for increased biogas production from thin stillage. J. Biotechnol. 2014, 175, 22–30.
- Dai, X.; Hu, C.; Zhang, D.; Dai, L.; Duan, N. Impact of a high ammonia-ammonium-pH system on methane-producing archaea and sulfate-reducing bacteria in mesophilic anaerobic digestion. Bioresour. Technol. 2017, 245, 598–605.
- Huertas, J.K.; Quipuzco, L.; Hassanein, A.; Lansing, S. Comparing hydrogen sulfide removal efficiency in a field-scale digester using microaeration and iron filters. Energies 2020, 13, 4793.
- Erdirencelebi, D.; Kucukhemek, M. Control of hydrogen sulphide in full-scale anaerobic digesters using iron (III) chloride: Performance, origin and effects. Water SA 2018, 44, 176–183.
- Sankaran, K.; Premalatha, M.; Vijayasekaran, M.; Somasundaram, V.T. Distillery wastewater treatment through anaerobic digestion and phycoremediation—A green industrial approach. Renew. Sustain. Energy Rev. 2014, 37, 634–643.
- Ruiz-Herrera, J.; Martínez, A.I.; Sentandreu, R. Determination of the stability of protein pools from the cell wall of fungi. Res. Microbiol. 2002, 153, 373–378.
- Lee, P.H.H.; Bae, J.; Kim, J.; Chen, W.-H. Mesophilic anaerobic digestion of corn thin stillage: A technical and energetic assessment of the corn-to-ethanol industry integrated with anaerobic digestion. J. Chem. Technol. Biotechnol. 2011, 86, 1514–1520.
- Schaefer, S.H.; Sung, S. Retooling the ethanol industry: Thermophilic anaerobic digestion of thin stillage for methane production and pollution prevention. Water Environ. Res. 2008, 80, 101–108.
- Oosterkamp, M.J.; Bauer, S.; Ibáñez, A.B.; Méndez-García, C.; Hong, P.; Cann, I.; Mackie, R.I. Identification of methanogenesis and syntrophy as important microbial metabolic processes for optimal thermophilic anaerobic digestion of energy cane thin stillage. Bioresour. Technol. Rep. 2019, 7, 100254.
- Andalib, M.; Hafez, H.; Elbeshbishy, E.; Nakhla, G.; Zhu, J. Treatment of thin stillage in a high-rate anaerobic fluidized bed bioreactor (AFBR). Bioresour. Technol. 2012, 121, 411–418.
- Sayedin, F.; Kermanshahi-Pour, A.; He, S. Anaerobic digestion of thin stillage of corn ethanol plant in a novel anaerobic baffled reactor. Waste Manag. 2018, 78, 541–552.
- Wilkinson, A.; Kennedy, K.J. Anaerobic digestion of corn ethanol thin stillage in batch and by high-rate down-flow fixed film reactors. Water Sci. Technol. 2012, 66, 1834–1841.
- Dereli, R.K.; van der Zee, F.P.; Heffernan, B.; Grelot, A.; van Lier, J.B. Effect of sludge retention time on the biological performance of anaerobic membrane bioreactors treating corn-to-ethanol thin stillage with high lipid content. Water Res. 2014, 49, 453–464.
- Melamane, X.L.; Tandlich, R.; Burgess, J.E. Treatment of wine distillery wastewater by high rate anaerobic digestion. Water Sci. Technol. 2007, 56, 9–16.
- Wolmarans, B.; de Villiers, G.H. Start-up of a UASB effluent treatment plant on distillery wastewater. Water SA 2002, 28, 63.
- Blonskaja, V.; Menert, A.; Vilu, R. Use of two-stage anaerobic treatment for distillery waste. Adv. Environ. Res. 2003, 7, 671–678.
- Cabrera-Díaz, A.; Reyes, I.P.; Merencio, D.O.; Lebrero, R.; Zaiat, M. Anaerobic digestion of sugarcane vinasse through a methanogenic UASB reactor followed by a packed bed reactor. Appl. Biochem. Biotechnol. 2017, 183, 1127–1145.
- Wu, B.; Lin, R.; Kang, X.; Deng, C.; Xia, A.; Dobson, A.D.W.; Murphy, J.D. Graphene addition to digestion of thin stillage can alleviate acidic shock and improve biomethane production. ACS Sustain. Chem. Eng. 2020, 8, 13248–13260.
- M’Arimi, M.M.; Zhang, Y.; Götz, G.; Kiriamiti, K.H.; Geißen, S.-U. Antimicrobial colorants in molasses distillery wastewater and their removal technologies. Int. Biodeterior. Biodegrad. 2014, 87, 34–43.
- Pereira, M.C.; Oliveira, D.A.; Hill, L.E.; Zambiazi, R.C.; Borges, C.D.; Vizzotto, M.; Mertens-Talcott, S.; Talcott, S.; Gomes, C.L. Effect of nanoencapsulation using PLGA on antioxidant and antimicrobial activities of guabiroba fruit phenolic extract. Food Chem. 2018, 240, 396–404.
- Elalami, D.; Carrere, H.; Abdelouahdi, K.; Garcia-Bernet, D.; Peydecastaing, J.; Vaca-Medina, G.; Oukarroum, A.; Zeroual, Y.; Barakat, A. Mild microwaves, ultrasonic and alkaline pretreatments for improving methane production: Impact on biochemical and structural properties of olive pomace. Bioresour. Technol. 2020, 299, 122591.
- Kaushik, A.; Basu, S.; Batra, V.; Balakrishnan, M. Fractionation of sugarcane molasses distillery wastewater and evaluation of antioxidant and antimicrobial characteristics. Ind. Crop. Prod. 2018, 118, 73–80.
- Fedorak, P.M.; Hrudey, S.E. The effects of phenol and some alkyl phenolics on batch anaerobic methanogenesis. Water Res. 1984, 18, 361–367.
- Chapleur, O.; Mazeas, L.; Godon, J.-J.; Bouchez, T. Asymmetrical response of anaerobic digestion microbiota to temperature changes. Appl. Microbiol. Biotechnol. 2015, 100, 1445–1457.
- Rosenkranz, F.; Cabrol, L.; Carballa, M.; Donoso-Bravo, A.; Cruz, L.; Ruiz-Filippi, G.; Chamy, R.; Lema, J. Relationship between phenol degradation efficiency and microbial community structure in an anaerobic SBR. Water Res. 2013, 47, 6739–6749.
- Hernandez, J.; Edyvean, R. Inhibition of biogas production and biodegradability by substituted phenolic compounds in anaerobic sludge. J. Hazard. Mater. 2008, 160, 20–28.
- Yadav, S.; Chandra, R. Biodegradation of organic compounds of molasses melanoidin (MM) from biomethanated distillery spent wash (BMDS) during the decolourisation by a potential bacterial consortium. Biodegradation 2012, 23, 609–620.
- Peña, M.; Coca, M.; González, G.; Rioja, R.; García, M. Chemical oxidation of wastewater from molasses fermentation with ozone. Chemosphere 2003, 51, 893–900.
- Cämmerer, B.; Jalyschkov, V.; Kroh, L.W. Carbohydrate structures as part of the melanoidin skeleton. Int. Congr. Ser. 2002, 1245, 269–273.
- Kaushik, A.; Basu, S.; Raturi, S.; Batra, V.; Balakrishnan, M. Recovery of antioxidants from sugarcane molasses distillery wastewater and its effect on biomethanation. J. Water Process. Eng. 2018, 25, 205–211.
- Nayak, J.K.; Amit; Ghosh, U.K. An innovative mixotrophic approach of distillery spent wash with sewage wastewater for biodegradation and bioelectricity generation using microbial fuel cell. J. Water Process. Eng. 2018, 23, 306–313.
- Prasad, R.K.; Srivastava, S. Electrochemical degradation of distillery spent wash using catalytic anode: Factorial design of experiments. Chem. Eng. J. 2009, 146, 22–29.
- Apollo, S.; Onyango, M.S.; Ochieng, A. An integrated anaerobic digestion and UV photocatalytic treatment of distillery wastewater. J. Hazard. Mater. 2013, 261, 435–442.
- Siles, J.; García-García, I.; Martín, A. Integrated ozonation and biomethanization treatments of vinasse derived from ethanol manufacturing. J. Hazard. Mater. 2011, 188, 247–253.
- Padoley, K.; Saharan, V.K.; Mudliar, S.; Pandey, R.; Pandit, A.B. Cavitationally induced biodegradability enhancement of a distillery wastewater. J. Hazard. Mater. 2012, 219–220, 69–74.
- Sangave, P.C.; Gogate, P.R.; Pandit, A.B. Ultrasound and ozone assisted biological degradation of thermally pretreated and anaerobically pretreated distillery wastewater. Chemosphere 2007, 68, 42–50.
- Melamane, X.; Tandlich, R.; Burgess, J. Anaerobic digestion of fungally pre-treated wine distillery wastewater. Afr. J. Biotechnol. 2007, 6, 1990–1993.
- Romero, C.; Brenes, M.; García, P.; García, A.; Garrido, A. Polyphenol changes during fermentation of naturally black olives. J. Agric. Food Chem. 2004, 52, 1973–1979.
- Tsioulpas, A.; Dimou, D.; Iconomou, D.; Aggelis, G. Phenolic removal in olive oil mill wastewater by strains of Pleurotus spp. in respect to their phenol oxidase (laccase) activity. Bioresour. Technol. 2002, 84, 251–257.
- Kumar, P.; Chandra, R. Decolourisation and detoxification of synthetic molasses melanoidins by individual and mixed cultures of Bacillus spp. Bioresour. Technol. 2006, 97, 2096–2102.
- Santal, A.R.; Singh, N.; Saharan, B.S. Biodegradation and detoxification of melanoidin from distillery effluent using an aerobic bacterial strain SAG5 of Alcaligenes faecalis. J. Hazard. Mater. 2011, 193, 319–324.
- Mohana, S.; Desai, C.; Madamwar, D. Biodegradation and decolourization of anaerobically treated distillery spent wash by a novel bacterial consortium. Bioresour. Technol. 2007, 98, 333–339.
- Sirianuntapiboon, S.; Zohsalam, P.; Ohmomo, S. Decolorization of molasses wastewater by Citeromyces sp. WR-43-6. Process. Biochem. 2004, 39, 917–924.
- Asgher, M.; Bhatti, H.N.; Ashraf, M.; Legge, R. Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation 2008, 19, 771–783.
- Li, W.; Li, X.; Zeng, K. Aerobic biodegradation kinetics of tannic acid in activated sludge system. Biochem. Eng. J. 2009, 43, 142–148.
- Lin, J.; Reddy, M.; Moorthi, V.; Qoma, B.E. Bacterial removal of toxic phenols from an industrial effluent. Afr. J. Biotechnol. 2008, 7, 2232–2238.
- Kanimozhi, R.; Vasudevan, N. Effect of organic loading rate on the performance of aerobic SBR treating anaerobically digested distillery wastewater. Clean Technol. Environ. Policy 2014, 16, 467–476.
- Anupama, S.; Pradeep, N.V.; Hampannavar, U.S. Anaerobic followed by aerobic treatment approaches for Spentwash using MFC and RBC. Sugar Tech 2013, 15, 197–202.
- Sundararaman, S.; Kumar, J.L.; Kumar, N.G. Reduction of COD and decolourisation of UASB spent wash using E-MBR. Res. J. Pharm. Technol. 2015, 8, 845–848.
- Satyawali, Y.; Balakrishnan, M. Treatment of distillery effluent in a membrane bioreactor (MBR) equipped with mesh filter. Sep. Purif. Technol. 2008, 63, 278–286.
- Gupta, R.; Satyawali, Y.; Batra, V.S.; Balakrishnan, M. Submerged membrane bioreactor using fly ash filters: Trials with distillery wastewater. Water Sci. Technol. 2008, 58, 1281–1284.
- Deschamps, L.; Merlet, D.; Lemaire, J.; Imatoukene, N.; Filali, R.; Clément, T.; Lopez, M.; Theoleyre, M.-A. Excellent performance of anaerobic membrane bioreactor in treatment of distillery wastewater at pilot scale. J. Water Process. Eng. 2021, 41, 102061.
- Travieso, L.; Benítez, F.; Sánchez, E.; Borja, R.; León, M.; Raposo, F.; Rincon, B. Assessment of a microalgae pond for post-treatment of the effluent from an anaerobic fixed bed reactor treating distillery wastewater. Environ. Technol. 2008, 29, 985–992.
- Ray, S.G.; Ghangrekar, M.M. Comprehensive review on treatment of high-strength distillery wastewater in advanced physico-chemical and biological degradation pathways. Int. J. Environ. Sci. Technol. 2019, 16, 527–546.
