You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Eduard Fernandez Nofrerias.
Given the technical simplicity of the bicaval valve implantation (CAVI) technique compared to other transcatheter devices, CAVI is postulated as a suitable alternative for a wide variety of patients affected with severe+ tricuspid regurgitation.
- Transcatheter Valves Therapies
- bicaval valve implantation
1. Introduction
Severe symptomatic tricuspid regurgitation (TR) is associated with poor short- to medium-term clinical outcomes, representing a leading cause of moderate-to-severe heart valve disease in developed countries [1,2][1][2]. Medical treatment and tricuspid valve (TV) surgery are the currently accepted therapies to treat severe symptomatic TR. However, despite TV surgical volume steadily increasing in the US, in-hospital mortality has remained static at 9%, suggestive of the fact that TV surgery is typically offered too late in the course of disease [3,4][3][4]. Several percutaneous devices have emerged during last decade to treat severe TR, with promising results in early feasibility studies, with pivotal randomized trials ongoing [5]. However, owing to its complex anatomy and challenges with peri-procedural imaging, many patients are still deemed unsuitable for these emerging percutaneous TV therapies that include edge-to-edge repair, direct annuloplasty and orthotopic valve replacement.
Caval valve implantation (CAVI) emerged initially as an alternative therapy for patients deemed as having ‘no other options’ for treating their severe symptomatic TR; such patients often afflicted with concomitant hepatic congestion and right heart failure [5]. Given the emerging challenges and applicability of many emerging percutaneous TV therapies to the broader cohort of severe TR candidates, the simplicity of CAVI underscores its attractiveness as an effective treatment option for many severe TR patients. CAVI has subsequently rapidly evolved with the development of dedicated devices adapted to the specific anatomy of the caval venous system [6,7][6][7].
2. TricValve®: Evidence, Current Studies, and Future Trials
Special access program for compassionate use of the dedicated TricValve® system has been granted in 11 countries. To date, 47 patients have been treated within this program, with an implantation success of 98%, with only 1 out of 47 patients suffering from device embolization requiring surgical correction. So far, in hospital mortality is 0% and 30-day mortality is 4%, comparable with other tricuspid percutaneous devices. Besides the global compassionate use program, there are currently 2 ongoing trials with specific protocols assessing the feasibility, safety and performance of the TricValve® system: TRICUS STUDY (NCT03723239), an early feasibility first-in-man trial; and TRICUS-EURO (NCT04141137), a CE mark trial. End-points also include assessment of the functional status, exercise capacity and quality of life. The 2 trials have single arm-an open label design and have completed patient enrollment. TRICUS included 9 patients from Lithuania, and TRICUS-EURO included 35 patients from Spain and Austria.| Inclusion Criteria | ||||
|---|---|---|---|---|
| Clinical |
| Hemodynamic |
| |
| Exclusion Criteria | ||||
| Clinical |
|
CABG: Coronary-Artery Bypass Graft. IVC: Inferior Vena Cava. LVEF: Left Ventricular Ejection Fraction. NYHA: New York Heart Association. OMT: Optimal Medical Therapy. PCI: Percutaneous Coronary Intervention. SVC: Superior Vena Cava. TR: Tricuspid Regurgitation. VKA: Vitamin K Antagonist.
3. TricValve®
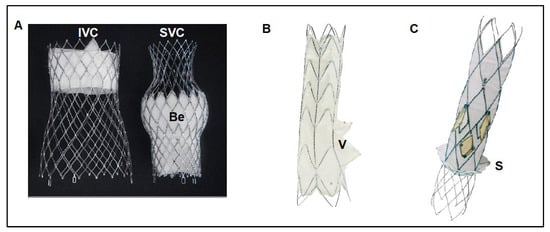
Currently, TricValve® is the only CAVI device with CE mark approval obtained in May 2021. The TricValve® implantation system consists of 2 TricValve® self-expanding nitinol structures with leaflets made out of bovine pericardium, one designed for the SVC and the other for the IVC–pre-mounted on a 27.5 F TricValve® Delivery System. The bioprosthetic leaflets are processed with anti-calcification treatment as well as chemical dehydration for preloading in the delivery system (Figure 1A). The bioprosthesis is specifically designed to adapt to the anatomic features of the cava, to minimize the risk or migration and para-valvular leaks, and it is available in 3 different sizes for the SVC (25, 29 and 33 mm) and 4 for the IVC (31, 35, 41 and 45 mm). Its caval fixation is based on stent design, radial force, and the degree of oversizing during implantation. The SVC device has a long skirt to prevent para-valvular leak covering the ½ of the inferior portion of the belly (Figure 1A). It has a high radial force within the belly portion of the device to fixate and seal, as well as within the valve segment to eliminate the risk of valve deformation and malfunction. The crown of the superior prosthesis has low radial force for valve stabilization and for improving alignment with the SVC. The inferior prosthesis has a short skirt to prevent hepatic vein occlusion, covering only the first 20 mm of the proximal edge. It has also a high strength nitinol frame in its proximal segment, where the valve is placed (Figure 1A), The inferior or anchoring segment of the inferior prosthesis has low radial force with large diameter to increase contact area with soft pressure upon the caval wall [6].
Figure 1. Current dedicated CAVI devices. (A) The TricValve® device consists of 2 self-expanding nitinol valvular stent frame devices specifically designed for the cava, one for the SVC and another for the IVC. The SVC device has a belly portion (Be) with high radial force to fix and seal the device into the SVC, has a long skirt that covers the 1/2 inferior parts of the device for preventing para-valvular leak. The valve is placed in the inferior part of the device. The IVC device has a short skirt only covering the first 20 mm of the proximal edge for preventing hepatic vein occlusion, and high radial force at the valve level for fixing the device and preventing valve distortion or malfunction. (B) Tricento® device is a customized device adapted to the anatomy of each particular patient. Device consists of a self-expanding covered stent frame placed into the SVC, right atrium and IVC with an opening valve (V) facing the tricuspid plane. (C) Trillium™ device is a cross-caval stent graft with multiple covered fenestrations arranged circumferentially around its right atrium portion. Trillium™ has a skirt at its inferior part to seal without occluding hepatic veins. Be: Belly part of TricValve® superior prosthesis. IVC: Inferior Vena Cava. SVC: Superior vena cava. S: Skirt of Trillium™ system. V: Valve of Tricento® system.
4. TricValve® Planning
Pre-implantation planning is based on clinical, hemodynamic and anatomical features. The system is designed for patients with at least severe TR. In summary, the TricValve® studies only included patients severely symptomatic (NYHA functional class III or IV or with previous admission for heart failure during last 12 months) despite optimal medical therapy, and deemed at high or prohibitive surgical risk for conventional tricuspid surgery. Pacemaker leads were not a contraindication. However, those with severe pulmonary hypertension (systolic pulmonary pressure >65 mmHg) or severe impaired right ventricular function (i.e., TAPSE < 13 mm) were contraindications. TricValve® requires a significant tricuspid V-wave to ensure adequate valve leaflet motion. Usually, a tricuspid V-wave is large in patients with severe TR. However, the tricuspid V-wave can be low in some circumstances even with severe TR, particularly if the RA is very large, or if right ventricular function is depressed, particularly when the intravascular volume is depleted. Tricvalve® should not be recommended if v-waves are <15 mmHg measured during a euvolumic setting.
Anatomically, bioprosthesis sizing requires accurate caval measurements on CT scanning to minimize the possibility of any safety issues and suboptimal performance results. Contrast-enhanced CT of the chest and abdomen, performed 70 to 85 s after injecting contrast agent into a peripheral vein is recommended for an uniform enhancement of both, SVC and IVC. An oversizing degree of 10 to 40% within the belly part for the SVC prosthesis and in the proximal part of the IVC prosthesis is desirable for optimizing fixation and seal. For the SVC, critical references are the diameter at the level of the confluence with the innominate trunk, the diameter at the level of pulmonary artery, diameter at the cavo-atrial junction, and the distance between these points. A minimum length of 50 mm between cavo-atrial junction and innominate confluence is needed for the SVC. The target position is to place the belly part of the SVC prosthesis, which has a long skirt covering the inferior half of the belly, at the level where the right pulmonary artery crosses the SVC. There should be caution in patients with short and tapered SVC, because risk of migration is higher in such anatomies. In these instances, a higher implant technique is recommended by placing the belly part of the SVC prosthesis between the confluence and the right pulmonary artery level. However, the belly part should not be positioned at the level of the confluence because of the risk of innominate occlusion. A high implantation technique is also recommended in patients with pacemaker or implantable cardioverter-defibrillator leads for minimizing the risk of migration.
For the IVC prosthesis, the critical points are diameters at the level of the junction with RA, below and above the hepatic veins, and the distance between supra-hepatic veins and cavo-atrial junction. Target position is to implant the proximal edge between the junction and the confluence of the supra-hepatic veins, entering into the atrium between 5 to 12 mm depending on the anatomy. The risk of peri-valvular leak increases if the proximal edge of the device enters within into the atrium more than 15 mm, since only the first 20 proximal mm of the device are covered by the skirt. Special caution is required in patients with a short distance between the supra-hepatic veins and the cavo-atrial junction, in patients with significant differences in diameter above and below the hepatic veins, or in patients with significant angulations between the cavo-atrial junction. In these cases, the contact between the device and the caval wall is less and this could affect device stability. To increase the attachment area, the valve should be positioned as low as possible (entering only 0–5 mm into the atrium). A very slow deployment is also recommended.
45. TricValve® Procedural Steps
The implantation requires 3 access sites (option of 4th): 1 right common femoral vein (27.5F) for device deployment and 2 left common femoral vein (5F and 7F) for pigtail control injections and a Swan-Ganz catheter. Optionally a 4th left basilic vein access (5F) can be useful for better identifying the confluence between innominate and SVC. Pre-closure with 1–2 Proglides in the right common femoral vein can be useful for hemostasis, but not mandatory. A Swan-Ganz catheter should be placed at the level of right pulmonary artery for reference. Using the pigtail (+ a multipurpose catheter within the innominate vein), an angiogram of the SVC with special attention to the confluence and the distance between the innominate vein ostium and the SVC at the level where the right branch of the pulmonary artery crosses it is recommended for planning the deployment (Figure 2A,B). A stiff wire placed in the subclavian or internal jugular vein is recommended for valve deployment. There are usually venous valves at the entrance to the subclavian or jugular vein. Careful handling with a multipurpose catheter may be necessary to cross these valves, often via simple catheter rotation as opposed to pushing. Deployment is undertaken by rotating the device’s roulette clockwise. Deployment should be performed slowly, starting in a high position and pulling down the system until it arrives at the target position (Figure 2C). It is not recommended to push the device for avoiding a venous wall pinching. The system is retrievable until the 80% is deployed. If we need to implant the device in a higher position we should re-sheath the system completely and re-start again in a higher position. Being a self-expanding device with a lot of radial force, the device trends to come out of the delivery system, so in general it is necessary to maintain tension during the deployment. Once the belly part of the prosthesis is deployed the systems remains very stable and we can release the tension. It is important to be sure that all 3 hooks attached to the device are released. Before removing the delivery system out of the patient, one should re-sheath it completely to avoid vascular complications.
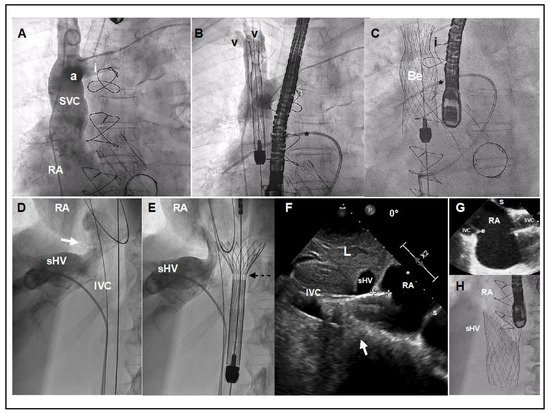

Figure 2. TricValve® implant details. (A) Venogram of the SVC with careful visualization of the innominate vein (i). The venogram was performed using a dual injection technique, 1 using a pig-tail through left femoral access, and another using a multipurpose through left basilica vein access. (B,C) Target position is to implant the belly part of the device (segment with higher radial force) at the level of pulmonary artery plane or between pulmonary artery plane and confluence with the innominate vein. A Swan-Ganz catheter placed into the right pulmonary artery and multipurpose catheter (or leads if patient has a pacemaker) may be a helpful reference. (D) Venogram with careful visualization of the right supra-hepatic vein confluence, IVC and junction with the right atrium (white arrow). (E) The first 20 mm of the proximal edge of the inferior valve is covered by a skirt to prevent occlusion of the hepatic vein. A radiolucent mark shows where the skirt of the valve ends (broken arrow). (F) TEE at 0º, showing the inferior prosthesis during deployment entering 11 mm into RA (between crosses). White arrow shows the cavo-atrial junction. Optimally positioning requires only 5 to 12 mm of the proximal portion should enter into the RA. (G) TEE bi-caval view after deployment of the superior and inferior prosthesis. (H) Angiographic view of the inferior vena cava prosthesis immediately after deployment. a: Azygous confluence. Be: Belly part of the superior vena cava prosthesis. e: Eustachian valve. I: Innominate vein. IVC: Inferior Vena Cava. L: Liver. RA: Right Atrium. sHV: superior Hepatic Vein. s: Septum. V: Vein valves of Subclavian and Jugular veins. Star: Right Pulmonary Artery with Swan Ganz.
For the IVC deployment, a venogram of the hepatic veins centered in the confluence with inferior cava and its junction with the RA is obtained, and serves as a reference for deployment (Figure 2D). The IVC prosthesis is deployed in a similar way to the SVC prosthesis, starting in a high position, into the right atrium, and pulling down the system at the same time that we are deploying the valve until our target position (proximal edge of the prosthesis landing in between the right atrium and supra-hepatic vein confluence) (Figure 2E). Depending on the anatomy or prosthesis size, a low implantation is typically recommended (as explained above). A TEE or transthoracic echocardiography can be useful, as the entrance or junction between IVC and right atrium is usually very well defined in the bi-caval view or sub-costal view. If echocardiographic images are available, a measure of the mm of the valve entering into the RA can be obtained during the deployment, as well as the presence of any leak (Figure 2F). The system is retrievable until 80% of the prosthesis is deployed. At this point the system remains very stable, then a release of tension is recommended for avoiding a jump effect when the knocks are untied. It is important to confirm that all 3 hooks attached to the device are released. The delivery system must be fully re-sheathed before removing it in order to avoid vascular complications.
References
- D’ARCY, J.L.; Coffey, S.; Loudon, M.A.; Kennedy, A.; Pearson-Stuttard, J.; Birks, J.; Frangou, E.; Farmer, A.J.; Mant, D.; Wilson, J.; et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: The OxVALVE Population Cohort Study. Eur. Heart J. 2016, 37, 3515–3522.
- Topilsky, Y.; Maltais, S.; Medina Inojosa, J.; Oguz, D.; Michelena, H.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovasc. Imaging 2019, 12, 433–442.
- Zack, C.J.; Fender, E.A.; Chandrashekar, P.; Reddy, Y.N.V.; Bennett, C.E.; Stulak, J.M.; Miller, V.M.; Nishimura, R.A. National Trends and Outcomes in Isolated Tricuspid Valve Surgery. J. Am. Coll. Cardiol. 2017, 70, 2953–2960.
- Kim, J.B.; Jung, S.-H.; Choo, S.J.; Chung, C.H.; Lee, J.W. Surgical outcomes of severe tricuspid regurgitation: Predictors of adverse clinical outcomes. Heart 2013, 99, 181–187.
- Asmarats, L.; Puri, R.; Latib, A.; Navia, J.L.; Rodés-Cabau, J. Transcatheter Tricuspid Valve Interventions. J. Am. Coll. Cardiol. 2018, 71, 2935–2956.
- Figulla, H.R.; Kiss, K.; Lauten, A. Transcatheter interventions for tricuspid regurgitation-heterotopic technology: TricValve. EuroIntervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2016, 12, Y116–Y118.
- Toggweiler, S.; De Boeck, B.; Brinkert, M.; Buhmann, R.; Bossard, M.; Kobza, R.; Cuculi, F. First-in-man implantation of the Tricento transcatheter heart valve for the treatment of severe tricuspid regurgitation. EuroIntervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2018, 14, 758–761.
More
