Antimicrobial peptides (AMPs) are naturally occurring molecules that are utilized as an innate immune response in a variety of organisms. AMPs can be constitutively expressed, and/or their transcription may be upregulated following pathogenic infection. Cecropins (Cecs) are insect AMPs, generally active against Gram-negative bacteria and to a lesser extent, Gram-positive bacteria. Some have been demonstrated to also exhibit an antifungal activity as well as anti-inflammatory and anti-tumor properties. Cecs function by associating their N- and C-terminal helices to the cellular membrane. Polar residues interact with the lipid phosphates while the non-polar residues burrow into the membrane. At high concentrations, Cecs form carpet-like structures with detergent-like properties that result in cell death. At low concentrations, Cecs organize into oligomers that form pores through the cellular phospholipid layer, resulting in an electrolyte imbalance that causes cell death.
- antimicrobial peptides
- Cecropins
- insects
- innate immunity
1. Introduction
Cecropins (Cecs) are one of the largest groups of insect AMPs and comprise Cecs and Cec-like peptides. In the absence of any infections, Cec genes can be constitutively expressed at low levels in different body compartments, as demonstrated in the Drosophila reproductive tract[1] or in the silkworm Bombyx mori midgut or fat body (a structure equivalent to the mammalian liver)[2]. Following an immune challenge, Cecs become highly transcribed in several tissues, such as gut epithelia or epidermis during local infections, and the fat body and hemocytes, during systemic infections (e.g., [1][2][3]). Like other AMPs, Cecs are translated as immature pre-peptides, undergo proteolytic cleavage of the N-terminal signal peptide, and are secreted in a mature and active form[4][5]. Before maturation, Cec sizes range between 58 and 79 aa, while active forms contain between 34 and 55 residues. Experimental and computational analyses indicated that Cec and Cec-like peptides are structurally related and are characterized by an N-terminal basic, amphipathic domain linked to a more hydrophobic C-terminal segment, through a flexible proline- and glycine-rich hinge region (Figure 1A[4][5][6]).
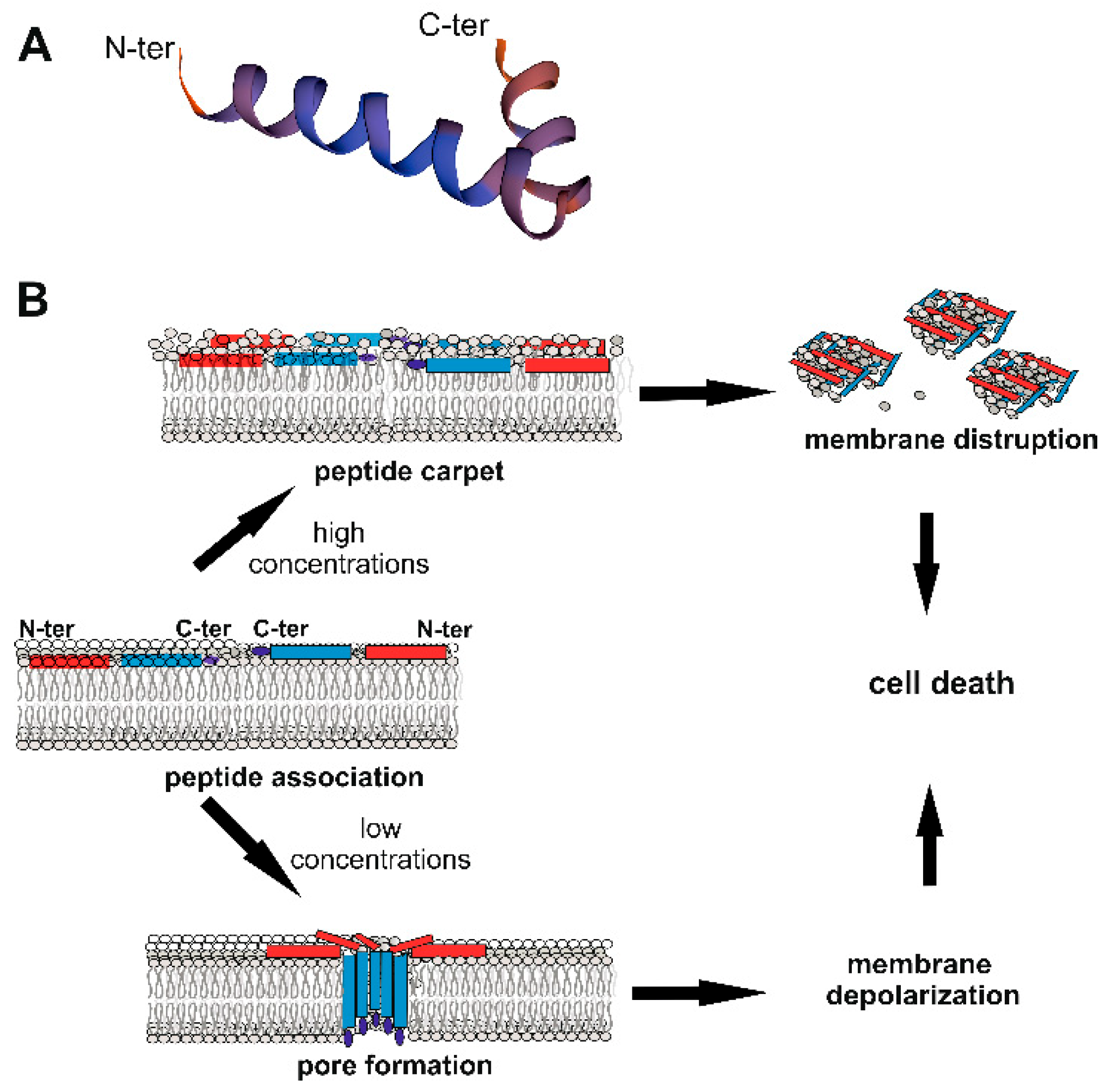
Figure 1. Cecropin (Cec) structure and mechanisms of action against bacteria. (A) Structure of the mature 35 aa B. mori Q53 Cec B natural variant[7] obtained using SWISS-MODEL (Available online: https://swissmodel.expasy.org/), showing N- and C-terminal α-helices linked through a flexible hinge region. (B) Model of action against bacteria. Cecs associate with the bacterial membrane, with the long axes of the α-helical domains parallel to the lipid bilayer surface. Polar residues interact with the lipid phosphates; non-polar residues bury in the hydrophobic core of the membrane. At high concentrations (upper part), Cecs form a carpet-like structure with detergent-like properties, disrupting membranes. At lower concentrations (lower part), Cecs form pores, which affect the cellular electrolyte balance, causing bacterial death[8]. The pore is formed of different Cec molecules organized as oligomers, with C-terminal hydrophobic domains submerged into the phospholipidic hydrophobic chains[9]. The red rectangle represents the N-terminal helix, the blue one the C-terminal helix; the dark blue ellipse indicates the C-terminal amidated residue.
2. Mechanism of Action Against Microorganisms
Insect Cecs and Cec-like peptides are generally active against Gram-negative bacteria and to a lesser extent, Gram-positive bacteria. Some have been demonstrated to also exhibit antifungal activity. Moreover, Cec and Cec-like peptides were shown to have a low toxicity against normal mammalian cells and a weak or absent hemolytic effect against mammalian erythrocytes. As for other cationic AMPs, the ability of these peptides to target microorganisms without interacting with host eukaryotic cells relies on the difference in composition of the respective cell membranes. Bacterial membranes are predominantly composed of negatively charged compounds (e.g., phosphatidylglycerol, cardiolipin, and phosphatidylserine), while eukaryotic membranes are positively charged by the presence of zwitterionic phospholipids and cholesterol[10]. Furthermore, Gram-negative bacteria possess an external membrane rich in negatively charged Lipopolysaccharides (LPS, also known as endotoxin), whereas in Gram-positive bacteria, the peptidoglycan is anchored to the cytoplasmic membrane by negatively charged teichoic acids. It is also generally thought that the discrimination between fungi and other eukaryotic host membranes is due to the different sterol compositions of their respective membranes[10].
Using chemically synthetized natural Cec variants and modified analogs, several studies have been performed to explain the Cec action mechanism against pathogens, as well as to identify the functions of specific residues within the peptide. Most mature Cec peptides contain a tryptophan residue in the first or second positions, which is considered important in conferring full antimicrobial activity to the peptide[4][5][6][11]. A study performed on Papiliocin, from the lepidopteran Papilio xuthus, suggested that the presence of tryptophan2 and phenylalanine5 aromatic residues in the N-terminal region are essential for the full-length peptide to interact with LPS in the outer membrane, and permeabilize the inner membrane of Gram-negative bacteria[12]. However, some dipteran Cecs, such as those from the black fly Simulium bannaense and the mosquito Aedes aegypti have been shown to be highly effective against different bacteria, although lacking an N-terminal tryptophan residue[13][14].
In several cases, Cec peptides undergo amidation of the C-terminal residue, a post-translational modification, which increases both antimicrobial activity and the action spectrum of the peptide[5][15]. It has been demonstrated that the antimicrobial activity of Cec AMPs relies on the structure they assume in the presence of bacterial cells. Circular dichroism analyses showed that in aqueous solution, Cecs have a random coiled structure but adopt α-helical conformations upon interaction with microbial membranes, where they exert a lytic effect[6][7][9][12]. Although some aspects remain unclear, it is currently accepted that Cec peptides do not interact with specific receptors but initially associate with the bacterial membrane along the axes of the α-helical domains parallel to the lipid bilayer surface. At this level, the polar residues of the peptide interact with the lipid phosphates, while the non-polar side chains burrow in the hydrophobic core of the membrane[6](Figure 2B). In a first model of action, the continuous accumulation of peptides at the bacterial lipid bilayer leads to the formation of a peptide “carpet” on the membrane surface. This “carpet” structure possesses intrinsic detergent-like lytic properties, which disintegrate the membranes[6]. Cec P1[16][17] and H. cecropia Cecs, when administrated at high concentrations (Cec P1 > 25 mM; H. cecropia Cecs > 5 mM), appear to act through this carpet-like mechanism (Figure 1B)[6][8]. However, at lower concentrations (2–5 µM), H. cecropia Cecs are able to associate with membranes and form channels or pores, which affect cellular electrolyte balance and in turn cause the death of the microorganism (Figure 1B)[6][8][9]. Initially, it was postulated that the N-terminal amphipathic regions of the peptides were involved in the formation of the pore (called “type II channel”), with the positively charged residues forming the inner channel[18][19]. Subsequent authors have hypothesized that the C-terminal hydrophobic domains of the peptides insert into the membrane giving rise to a more stable pore (type I channel), in which the polar aa of the C-terminal helices are oriented toward the center of the pore[8][9][19]. Efimova and colleagues analyzed the effect of H. cecropia Cecs A and B in model lipid membranes, with or without small molecules capable of modifying the membrane physical-chemical properties[8][9]. Using these data, they developed a model in which Cec peptides first interact as monomers with the hydrophilic heads of the lipid bilayer surface, acting parallel to the membrane plane. Next, the peptides submerge their C-terminal hydrophobic domains into the phospholipidic hydrophobic chain. Individual Cec molecules then organize into oligomers forming ion-permeable pores in the cell membrane (Figure 1B). Other monomers can then insert into the pores, increasing the ion channels’ conductance. The authors also postulated that all the steps of this process are reversible and in equilibrium[9]. This pore model therefore resembles the “barrel-stave” model, in which the different C-terminal regions of the H. cecropia Cec peptides are organized to form a barrel penetrating the bacterial membrane. However, in cases where the peptide is shorter than ~ 22 aa (e.g., synthetically Cec-derived analogs), the structure of the pore might be more similar to the so-called “toroidal-pore” model, in which the pore is composed by both peptides and lipids[6].
As mentioned above, natural Cec and Cec-like peptides show a higher activity against Gram-negative compared to Gram-positive bacteria. This feature has been related to the difference in the intrinsic properties of bacterial membranes (i.e., lipid composition, charge density, and electrochemical potential across the membrane), as demonstrated when evaluating H. cecropia Cec B against protoplasts obtained from Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus or S. epidermidis[20]. Moreover, a recent study on natural Papiliocin and its modified derivatives associated the Cec’s preferential activity against Gram-negative bacteria specifically with the presence of the C-terminal helix. In fact, compared to the full-length natural form, a truncated Papiliocin carrying only the N-terminal portion was less effective against Gram-negative, and more active against Gram-positive bacteria[12].
Finally, in a study evaluating the interaction between different B. mori natural Cec B variants and live Gram-negative Pseudomonas aeruginosa, it was suggested that Cecs might first affect the outer bacterial membrane, enabling the translocation of the peptide to the inner membrane, resulting in the disorganization of both lipid bilayers[7].
This entry is an excerpt from the open-access article:
Brady, D., Grapputo, A., Romoli, O., & Sandrelli, F. (2019). Insect Cecropins, Antimicrobial Peptides with Potential Therapeutic Applications. International journal of molecular sciences, https://doi.org/10.3390/ijms20235862
References
- Hanna Uvell; Ylva Engström; A multilayered defense against infection: combinatorial control of insect immune genes. Trends in Genetics 2007, 23, 342-349, 10.1016/j.tig.2007.05.003.
- Ottavia Romoli; Alessio Saviane; Andrea Bozzato; Paola D’Antona; Gianluca Tettamanti; Andrea Squartini; Silvia Cappellozza; Federica Sandrelli; Differential sensitivity to infections and antimicrobial peptide-mediated immune response in four silkworm strains with different geographical origin.. Scientific Reports 2017, 7, 1048, 10.1038/s41598-017-01162-z.
- Wanying Yang; Tingcai Cheng; Mingqiang Ye; Xiaojuan Deng; Huiyu Yi; Yadong Huang; Xiang Tan; Ng Han; Bo Wang; ZhongHuai Xiang; et al.Yang CaoQingYou Xia Functional Divergence among Silkworm Antimicrobial Peptide Paralogs by the Activities of Recombinant Proteins and the Induced Expression Profiles. PLOS ONE 2011, 6, e18109, 10.1371/journal.pone.0018109.
- Qinghua Wu; Jiří Patočka; Kamil Kuča; Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461, 10.3390/toxins10110461.
- Hui-Yu Yi; Munmun Chowdhury; Ya-Dong Huang; Xiao-Qiang Yu; Insect antimicrobial peptides and their applications.. Applied Microbiology and Biotechnology 2014, 98, 5807-22, 10.1007/s00253-014-5792-6.
- Hiromi Sato; Jimmy B. Feix; Peptide–membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochimica et Biophysica Acta (BBA) - Biomembranes 2006, 1758, 1245-1256, 10.1016/j.bbamem.2006.02.021.
- Ottavia Romoli; Shruti Mukherjee; Sk Abdul Mohid; Arkajyoti Dutta; Aurora Montali; Elisa Franzolin; Daniel Brady; Francesca Zito; Elisabetta Bergantino; Chiara Rampazzo; et al.Gianluca TettamantiAnirban BhuniaFederica Sandrelli Enhanced Silkworm Cecropin B Antimicrobial Activity against Pseudomonas aeruginosa from Single Amino Acid Variation. ACS Infectious Diseases 2019, 5, 1200-1213, 10.1021/acsinfecdis.9b00042.
- Svetlana S. Efimova; Ludmila V. Schagina; Olga S. Ostroumova; Channel-Forming Activity of Cecropins in Lipid Bilayers: Effect of Agents Modifying the Membrane Dipole Potential. Langmuir 2014, 30, 7884-7892, 10.1021/la501549v.
- S. S. Efimova; R. Ya. Medvedev; E. G. Chulkov; L. V. Schagina; O. S. Ostroumova; Regulation of the Pore-Forming Activity of Cecropin A by Local Anesthetics. Cell and Tissue Biology 2018, 12, 331-341, 10.1134/s1990519x18040028.
- Yeaman M.R., Yount N.Y.; Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003, 55, 27-55, 10.1124/pr.55.1.2 .
- David Andreu; R. B. Merrifield; Haakan Steiner; Hans G. Boman; N-Terminal analogs of cecropin A: synthesis, antibacterial activity, and conformational properties. Biochemistry 1985, 24, 1683-1688, 10.1021/bi00328a017.
- Eunjung Lee; Jin-Kyoung Kim; Dasom Jeon; Ki-Woong Jeong; Areum Shin; Yangmee Kim; Functional Roles of Aromatic Residues and Helices of Papiliocin in its Antimicrobial and Anti-inflammatory Activities. Scientific Reports 2015, 5, 12048, 10.1038/srep12048.
- Jing Wu; Lixian Mu; Li Zhuang; Yi Han; Tong Liu; Jun Li; Yuan Yang; Hailong Yang; Lin Wei; A cecropin-like antimicrobial peptide with anti-inflammatory activity from the black fly salivary glands.. Parasites & Vectors 2015, 8, 561, 10.1186/s13071-015-1176-8.
- Elamparithi Jayamani; Rajmohan Rajamuthiah; Jonah Larkins-Ford; Beth Burgwyn Fuchs; Annie L. Conery; Andreas Vilcinskas; Frederick M. Ausubel; Eleftherios Mylonakis; Insect-Derived Cecropins Display Activity against Acinetobacter baumannii in a Whole-Animal High-Throughput Caenorhabditis elegans Model. Antimicrobial Agents and Chemotherapy 2015, 59, 1728-1737, 10.1128/AAC.04198-14.
- Miray Tonk; Andreas Vilcinskas; The Medical Potential of Antimicrobial Peptides from Insects. Current Topics in Medicinal Chemistry 2016, 17, 554-575, 10.2174/1568026616666160713123654.
- J. Y. Lee; A. Boman; C. X. Sun; Mats Andersson; H. Jörnvall; V. Mutt; H. G. Boman; Antibacterial peptides from pig intestine: isolation of a mammalian cecropin.. Proceedings of the National Academy of Sciences 1989, 86, 9159-9162, 10.1073/pnas.86.23.9159.
- M. Andersson; A. Boman; H. G. Boman; Ascaris nematodes from pig and human make three antibacterial peptides: isolation of cecropin P1 and two ASABF peptides.. Cellular and Molecular Life Sciences 2003, 60, 599-606, 10.1007/s000180300051.
- B. Christensen; J. Fink; R. B. Merrifield; D. Mauzerall; Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes.. Proceedings of the National Academy of Sciences 1988, 85, 5072-5076, 10.1073/pnas.85.14.5072.
- S.R. Durell; G. Raghunathan; H.R. Guy; Modeling the ion channel structure of cecropin.. Biophysical Journal 1992, 63, 1623-1631, 10.1016/s0006-3495(92)81730-7.
- A J Moore; W D Beazley; M C Bibby; D A Devine; Antimicrobial activity of cecropins.. Journal of Antimicrobial Chemotherapy 1996, 37, 1077–1089, 10.1093/jac/37.6.1077.
