In 1924, to understand the processes involved in developmental biology, Spemann and Mangold transplanted a blastopore lip between different ectodermal regions of amphibian embryos. The transplanted dorsal tissue differentiated mostly into a notochord, while the ectoderm of the host dorsal tissue that was sitting above the transplanted region (blastopore lip) was induced and differentiated to form a Siamese twin containing dorsal tissues such as somites and a neural plate, which would form the central nervous system, forming the bulk of a second axis. The major findings were that the transplant had altered the fate of the overlying cells and that the neural folds were built from recipient cells and not donor cells. Spemann and Mangold discovered the organizing center in the dorsal blastopore lip of amphibians. This center consists of a cluster of cells in the developing embryo that have the ability to interact and instruct morphogenesis in the surrounding cells during gastrulation. When transplanted to the ventral side of the embryo, the center will induce the formation of a secondary axis, promoting the development of the central nervous system, organs, and tissues, as well as the formation of the main body axis. Spemann and Mangold found the first evidence of the organizing center, thereafter called the “Spemann organizer”, and its major role in the development of vertebrates. This discovery also introduced the concept of induction in embryonic development, which refers to the method used by specific cells to affect the fate of other embryonic cells. A major milestone had been achieved for developmental biology.
- spemann organizer
1. Introduction
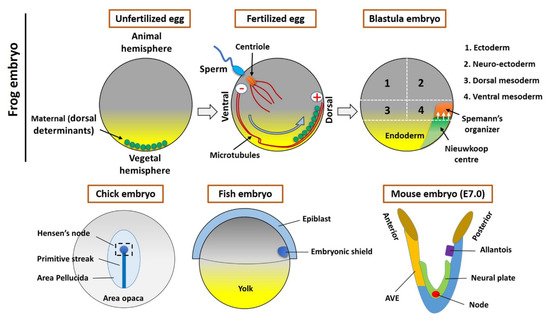
2. The Spemann’s Organizer and Homologous Tissue in Vertebrates
2.1. The Maternal Determinants Establish the Organizer
2.2. The Homologous Structure of Organizer in Other Vertebrates
References
- Spemann, H.; Mangold, H. über Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Arch. Für Mikrosk. Anat. Und Entwickl. 1924, 100, 599–638.
- De Robertis, E.M. Spemann’s organizer and self-regulation in amphibian embryos. Nat. Rev. Mol. Cell Biol. 2006, 7, 296–302.
- Lemaire, P.; Kodjabachian, L. The vertebrate organizer: Structure and molecules. Trends Genet. 1996, 12, 525–531.
- Anderson, C.; Khan, M.A.F.; Wong, F.; Solovieva, T.; Oliveira, N.M.M.; Baldock, R.A.; Tickle, C.; Burt, D.W.; Stern, C.D. A strategy to discover new organizers identifies a putative heart organizer. Nat. Commun. 2016, 7, 12656.
- Thisse, B.; Thisse, C. Formation of the vertebrate embryo: Moving beyond the Spemann organizer. Semin. Cell Dev. Biol. 2015, 42, 94–102.
- Harland, R.; Gerhart, J. Formation and function of Spemann’s organizer. Annu. Rev. Cell Dev. Biol. 1997, 13, 611–667.
- Moriyama, Y.; De Robertis, E.M. Embryonic regeneration by relocalization of the Spemann organizer during twinning in Xenopus. Proc. Natl. Acad. Sci. USA 2018, 115, E4815–E4822.
- Garcia-Fernandez, J.; D’ Aniello, S.; Escriva, H. Organizing chordates with an organizer. Bioessays 2007, 29, 619–624.
- Xanthos, J.B.; Kofron, M.; Tao, Q.; Schaible, K.; Wylie, C.; Heasman, J. The roles of three signaling pathways in the formation and function of the Spemann Organizer. Development 2002, 129, 4027–4043.
- De Robertis, E.M.; Larrain, J.; Oelgeschlager, M.; Wessely, O. The establishment of Spemann’s organizer and patterning of the vertebrate embryo. Nat. Rev. Genet. 2000, 1, 171–181.
- Carron, C.; Shi, D.L. Specification of anteroposterior axis by combinatorial signaling during Xenopus development. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 150–168.
- Martinez Arias, A.; Steventon, B. On the nature and function of organizers. Development 2018, 145, dev159525.
- Rodriguez, T.A.; Srinivas, S.; Clements, M.P.; Smith, J.C.; Beddington, R.S. Induction and migration of the anterior visceral endoderm is regulated by the extra-embryonic ectoderm. Development 2005, 132, 2513–2520.
- Leikola, A. Hensen’s node—the ‘organizer’ of the amniote embryo. Experientia 1976, 32, 269–277.
- Niehrs, C. Regionally specific induction by the Spemann-Mangold organizer. Nat. Rev. Genet. 2004, 5, 425–434.
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999.
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473.
- Chang, L.S.; Kim, M.; Glinka, A.; Reinhard, C.; Niehrs, C. The tumor suppressor PTPRK promotes ZNRF3 internalization and is required for Wnt inhibition in the Spemann organizer. Elife 2020, 9, e51248.
- Ding, Y.; Ploper, D.; Sosa, E.A.; Colozza, G.; Moriyama, Y.; Benitez, M.D.; Zhang, K.; Merkurjev, D.; De Robertis, E.M. Spemann organizer transcriptome induction by early beta-catenin, Wnt, Nodal, and Siamois signals in Xenopus laevis. Proc. Natl. Acad. Sci. USA 2017, 114, E3081–E3090.
- Schohl, A.; Fagotto, F. A role for maternal beta-catenin in early mesoderm induction in Xenopus. EMBO J. 2003, 22, 3303–3313.
- Wessely, O.; Kim, J.I.; Geissert, D.; Tran, U.; De Robertis, E.M. Analysis of Spemann organizer formation in Xenopus embryos by cDNA macroarrays. Dev. Biol. 2004, 269, 552–566.
- Gritsman, K.; Talbot, W.S.; Schier, A.F. Nodal signaling patterns the organizer. Development 2000, 127, 921–932.
- Nieto, M.A. Reorganizing the organizer 75 years on. Cell 1999, 98, 417–425.
- Sudou, N.; Yamamoto, S.; Ogino, H.; Taira, M. Dynamic in vivo binding of transcription factors to cis-regulatory modules of cer and gsc in the stepwise formation of the Spemann-Mangold organizer. Development 2012, 139, 1651–1661.
- Takahashi, S.; Yokota, C.; Takano, K.; Tanegashima, K.; Onuma, Y.; Goto, J.; Asashima, M. Two novel nodal-related genes initiate early inductive events in Xenopus Nieuwkoop center. Development 2000, 127, 5319–5329.
- Agius, E.; Oelgeschlager, M.; Wessely, O.; Kemp, C.; De Robertis, E.M. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development 2000, 127, 1173–1183.
- Wittler, L.; Kessel, M. The acquisition of neural fate in the chick. Mech. Dev. 2004, 121, 1031–1042.
- Shih, J.; Fraser, S.E. Characterizing the zebrafish organizer: Microsurgical analysis at the early-shield stage. Development 1996, 122, 1313–1322.
- Xiao, C.; Nitsche, F.; Bazzi, H. Visualizing the Node and Notochordal Plate In Gastrulating Mouse Embryos Using Scanning Electron Microscopy and Whole Mount Immunofluorescence. J. Vis. Exp. 2018, 9, e58321.
- Tam, P.P.; Behringer, R.R. Mouse gastrulation: The formation of a mammalian body plan. Mech. Dev. 1997, 68, 3–25.
- Satoh, N.; Tagawa, K.; Takahashi, H. How was the notochord born? Evol. Dev. 2012, 14, 56–75.
- Agathon, A.; Thisse, C.; Thisse, B. The molecular nature of the zebrafish tail organizer. Nature 2003, 424, 448–452.
- Stuhlmiller, T.J.; Garcia-Castro, M.I. Current perspectives of the signaling pathways directing neural crest induction. Cell. Mol. Life Sci. 2012, 69, 3715–3737.
- Stemple, D.L. Structure and function of the notochord: An essential organ for chordate development. Development 2005, 132, 2503–2512.
- Darras, S.; Nishida, H. The BMP signaling pathway is required together with the FGF pathway for notochord induction in the ascidian embryo. Development 2001, 128, 2629–2638.
