The ongoing SARS-CoV-2 pandemic is a serious threat to public health worldwide and, to date, no effective treatment is available. Thus, we herein review the pharmaceutical approaches to SARS-CoV-2 infection treatment. Numerous candidate medicines that can prevent SARS-CoV-2 infection and replication have been proposed. These medicines include inhibitors of serine protease TMPRSS2 and angiotensin converting enzyme 2 (ACE2). The S protein of SARS-CoV-2 binds to the receptor in host cells. ACE2 inhibitors block TMPRSS2 and S protein priming, thus preventing SARS-CoV-2 entry to host cells. Moreover, antiviral medicines (including the nucleotide analogue remdesivir, the HIV protease inhibitors lopinavir and ritonavir, and wide-spectrum antiviral antibiotics arbidol and favipiravir) have been shown to reduce the dissemination of SARS-CoV-2 as well as morbidity and mortality associated with COVID-19
- therapy
- SARS-CoV-2
- combination therapy
- virus-based therapy
- host-based therapy
- SARS-CoV-2 cell entry inhibitors
1. Introduction
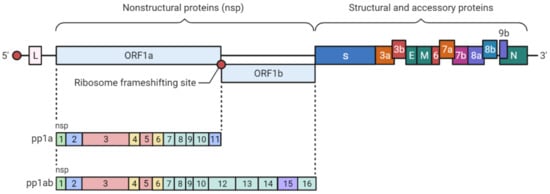
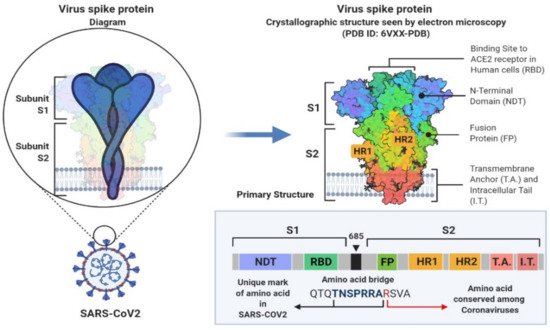
2. SARS-CoV-2 Genetic Structure and Pathogenic Mechanism
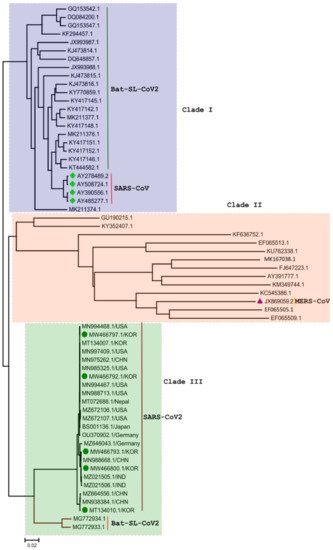
3. Phylogenetic Analysis of SARS-CoV-2 Genome
4. Therapeutic Approaches to SAR-COV-2 Infection
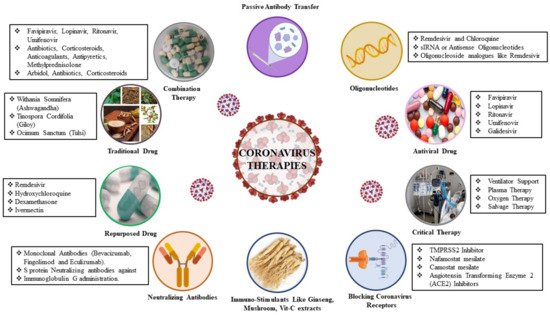
4.1. Virus-Based Therapy
| Antiviral Agent | Drug Target | Mechanism of Action | Infectious Disease | References |
|---|---|---|---|---|
| Remdesivir | RdRp | Terminates the non-obligate chain | SARS-CoV-2, MERS-CoV, SARS-CoV |
| S.N. | Antibody Name | Antibody Type | Origin | PDB ID | Epitopes | Neutralizing Mechanism | Cross Neutralizing Activity | Protective Efficacy | Ref | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [ | 20 | ] | |||||||||||||||||
| Baricitinib | Clathrin-mediated endocytosis | [ | 34 | ] | Favipiravir | RdRp | Inhibits RdRp | SARS-CoV-2, Influenza | |||||||||||
| 1 | CV30 | Human IgG | Infected COVID-19 patients | 6XE1 | D420-Y421, Y453, L455-N460, Y473-S477, F486-N487, Y489, Q493, T500, G502, Y505 | Block hACE2-RBD interaction | no | IC50 value of 0.03 µg/mL | [35 | ||||||||||
| [ | 21 | ] | |||||||||||||||||
| ] | Chloroquine | Endosomal acidification |
A lysosomatropic base that appears to disrupt intracellular trafficking and viral fusion events | SARS-CoV-2, SARS-CoV, MERS-CoV | [33] | ||||||||||||||
| 2 | REGN10933 Recombinant | full-human antibodies | Humanized mice and COVID-19-convalescent patients | 6XDG | R403, K417, Y421, Y453, L455-F456, A475-G476, E484-Y489, Q493 | Block hACE2-RBD interaction, ADCC & ADCP | no | IC50 value of 37.4 pM | siRNA | RdRp | Short chains of dsRNA that interfere | ||||||||
| Convalescent plasma | - | SARS-CoV, MERS-CoVWu | |||||||||||||||||
| 3 | Inhibits virus entry to the target cells | [ | B38 | 22] | |||||||||||||||
| SARS-CoV, SARS-CoV-2, Influenza | [ | 35 | , | [35 | 36] | ][36 | Human IgG | ] | COVID-19-convalescent patient | 7BZ5 | R403, D405-E406, Q409, D420-Y421, Y452, L454-N460, Y473-S477, F486-N487, Y489-F490, Q493-G496, Q498, T500-V503, Y505 | Block hACE2-RBD interaction | no | A single dose of B38 (25 mg/kg) | [35 | Galidesivir | RdRp | Inhibits viral RNA polymerase function by | Galidesivir SARS-CoV-2, |
| ] | Camostat Mesylate | Surface protease | Potent serine protease inhibitor | [23] | |||||||||||||||
| SARS-CoV, MERS-CoV, HcoV-229E | [ | ||||||||||||||||||
| 4 | CC12.1 | 33 | ] | Human IgG | COVID-19-convalescent patient | 6XC3 | R403, D405-E406, R408-Q409, D420-Y421, Y453, L455-N460, Y473-S477, F486-N487, Y489, Q493-G496, Q498, T500-V503, Y505 | Block hACE2-RBD interaction | no | IC50 value of 0.019 µg/mL | [36] | Ribavirin | RdRp | Inhibits viral RNA synthesis and mRNA capping | SARS-CoV-2, MERS-CoV, SARS-CoV, | ||||
| Corticosteroids | Pulsed methylprednisolone | Patients with severe MERS who were treated with systemic corticosteroid with or without antivirals and interferons had no favorable response | SARS-CoV, MERS-CoVL | [24] | |||||||||||||||
| [ | 35 | ] | |||||||||||||||||
| 5 | CB6 | Human IgG | COVID-19-convalescent patient | 7C01 | R403, D405-E406, R408-Q409, D420-Y421, L455-N460, Y473-S477, F486-N487, Y489, Q493, Y495, N501-G502, G504-Y505 | LJ001 and JL103 | Lipid membrane | ||||||||||||
| Block hACE2-RBD interaction | no | A single dose of CB6-LALA (50 mg/kg) | [ | 37 | ] | Nitazoxanide | Interferon responseMembrane-binding photosensitizers that induce | Enveloped viruses (IAV, filoviruses, poxviruses, arenaviruses, bunyaviruses, paramyxoviruses, flaviviruses and HIV-1) | [25] | ||||||||||
| Induces the host innate immune response | |||||||||||||||||||
| 6 | Coronaviruses, SARS-CoV-2 | [ | C105 | Human IgG | 19] | COVID-19-convalescent patient | 6XCN, 6XCM | R403, D405, R408, D420-Y421, Y453, L455-N460, Y473, A475-G476, F486-N487, G502, Y505 | Block hACE2-RBD interaction | no | IC50 value of 26.1 ng/mL | [41] | CR3022 | Spike glycoprotein | Immunogenic antigen against Spike protein | ||||
| Recombinant interferons | Interferon response | SARS-CoV-2, SARS-CoV | Exogenous interferons | [26] | |||||||||||||||
| SARS-CoV-2, SARS-CoV, MERS-CoV | [ | 37 | ] | ||||||||||||||||
| 7 | CC12.3 | Human IgG | COVID-19-convalescent patient | 6XC7 | R403, D405, D420-Y421, Y453, L455-N460, Y473-S477, F486-N487, Y489, Q493, G496, N501, Y505 | Block hACE2-RBD interaction | no | IC50 value of 0.018 µg/mL | [42] | Griffithsin | Spike glycoprotein | ||||||||
| 8 | Griffithsin binds to the SARSCoV-2 spike | CR3022 | SARS-CoV-2 | [27] | |||||||||||||||
| Human IgG | SARS-convalescent patient | 6YOR, 6 W41 | Y369-N370, F374-K386, L390, F392, D428, T430, F515-L517 | Trapping RBD in the less stable up conformation while leading to destabilization of S | SARS-CoV, SARS-CoV-2 | ND50 value of 0.114 µg/mL | [ | 19 | ] | Peptide (P9) | Spike glycoprotein | Inhibits spike protein-mediated cell-cell entry or | Broad-spectrum (SARS-CoV, MERS-CoV, influenza) | [28 | |||||
| 9 | EY6A | Human IgG | Late-stage COVID-19 patient | ] | |||||||||||||||
| 6ZDH, 6ZER, 6ZCZ | Y369, F374-S375, F377-K386, N388, L390, P412-G413, D427-F429, L517 | destabilization of S | SARS-CoV, SARS-CoV-2 | ND50 value of ~10.8 µg/mL | [ | 26 | ] | Nafamostat | Spike glycoprotein | Inhibits spike-mediated membrane fusion A | SARS-CoV-2, MERS-CoV | [29] | |||||||
| Ritonavir | 3CLpro | Inhibits 3CLpro | SARS-CoV-2, MERS-CoV | [30] | |||||||||||||||
| Lopinavir | 3CLpro | Inhibits 3CLpro | SARS-CoV-2, MERS-CoV, SARS-CoV, HCoV-229E, HIV, HPV | [31] | |||||||||||||||
| Darunavir and cobicistat | 3CLpro | Inhibits 3CLpro | SARS-CoV-2 | [32] |
4.2. Host-Based Therapy
| Antiviral Agent | Drug Target | Mechanism of Action | Infectious Disease | References |
|---|---|---|---|---|
| Baricitinib | Clathrin-mediated endocytosis |
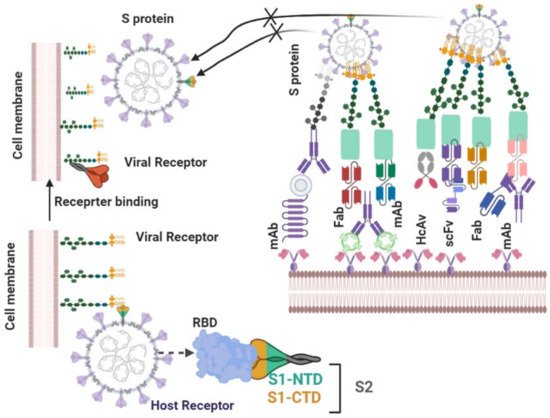
4.2.1. Neutralizing Antibodies
| 10 | |||||||||
| VHH-72 | |||||||||
| Llama single domain antibody | |||||||||
| llama immunized with prefusionstabilized betacoronavirus spikes | |||||||||
| 6WAQ | Y356-T359, F361-C366, A371-T372, G391-D392, R395, N424, I489, Y494 | Trapping RBD in the less stable up conformation while leading to destabilization of S, Block hACE2_RBD interaction | SARS-CoV, SARS-Co-V-2 | IC50 values of 0.14 µg/mL and 0.2 mg/mL. | [ | 19 | ] | ||
| 11 | BD23 | Human IgG | COVID-19-convalescent patient | 7BYR | G446, Y449, L452, T470, E484-F486, Y489-F490, L492-S494, G496, Q498, T500-N501, Y505 | Block hACE-RBD2 interaction | no | IC50 value of 8.5 µg/mL | [26] |
| 12 | Fab 2–4 | Human IgG | Infected COVID-19 patients | 6XEY | Y449, Y453, L455-F456, E484-F486, Y489-F490, L492-S494, G496 | Locking RBD in the down conformation while occluding access to ACE2 | no | Neutralizing SARS-CoV-2 live virus with IC50 value of 0.057 µg/mL | [41] |
References
- Nadeem, M.S.; Zamzami, M.A.; Choudhry, H.; Murtaza, B.N.; Kazmi, I.; Ahmad, H.; Shakoori, A.R. Origin, potential therapeutic targets and treatment for coronavirus disease (COVID-19). Pathogens 2020, 9, 307.
- Zhang, Y.Z.; Holmes, E.C. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell 2020, 181, 223–227.
- Lai, C.C.; Liu, Y.H.; Wang, C.Y.; Wang, Y.H.; Hsueh, S.C.; Yen, M.Y.; Ko, W.C.; Hsueh, P.R. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. J. Microbiol. Immunol. Infect. 2020, 53, 404–412.
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848.
- Tian, Y.; Rong, L.; Nian, W.; He, Y. Review article: Gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020, 51, 843–851.
- Davies, N.G.; Klepac, P.; Liu, Y.; Prem, K.; Jit, M.; CMMID COVID-19 Working Group; Eggo, R.M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020, 26, 1205–1211.
- Khan, S.; Siddique, R.; Shereen, M.A.; Ali, A.; Liu, J.; Bai, Q.; Bashir, N.; Xue, M. Emergence of a novel Coronavirus, severe acute respiratory syndrome Coronavirus 2: Biology and therapeutic options. J. Clin. Microbiol. 2020, 58, e00187-20.
- Singh, D.D.; Jain, A. Multipurpose Instantaneous Microarray Detection of Acute Encephalitis Causing Viruses and Their Expression Profiles. Curr. Microbiol. 2012, 65, 290–303.
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor recognition by the novel Coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20.
- Ton, A.T.; Gentile, F.; Hsing, M.; Ban, F.; Cherkasov, A. Rapid identification of potential inhibitors of SARS-CoV-2 main protease by deep docking of 1.3 billion compounds. Mol. Inform. 2020, 10, 202000028.
- Pan, H.; Peto, R.; Henao-Restrepo, A.-M.; Karim, Q.A.; Alejandria, M.; García, C.H.; Kieny, M.-P.; Malekzadeh, R.; Murthy, S.; Preziosi, M.-P.; et al. Repurposed antiviral drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511.
- Lam, T.T.-Y.; Shum, M.H.-H.; Zhu, H.-C.; Tong, Y.-G.; Ni, X.-B.; Liao, Y.-S.; Wei, W.; Cheung, W.Y.-M.; Li, W.-J.; Li, L.-F.; et al. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature 2020, 583, 282–285.
- Khateeb, J.; Li, Y.; Zhang, H. Emerging SARS-CoV-2 variants of concern and potential intervention approaches. Crit. Care 2021, 25, 244.
- Monteil, V.; Kwon, H.; Patricia, P.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado del Pozo, C.; et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell Press 2020, 181, 905–913.
- Zhou, Y.; Hou, Y.; Shen, J.; Huang, Y.; Martin, W.; Cheng, F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020, 6, 14–18.
- Rabaan, A.A.; Al-Ahmed, S.H.; Haque, S.; Sah, R.; Tiwari, R.; Malik, Y.S.; Dhama, K.; Yatoo, M.I.; Bonilla-Aldana, D.K.; RodriguezMorales, A.J.; et al. SARS-CoV-2, SARS-CoV, and MERS-CoV: A comparative overview. Infez. Med. 2020, 28, 174–184.
- Ko, M.; Chang, S.; Byun, S.; Ianevski, A.; Choi, I.; D’Orengiani, A.-L.P.H.D.; Ravlo, E.; Wang, W.; Bjørås, M.; Kainov, D.; et al. Screening of FDA-Approved Drugs Using a MERS-CoV Clinical isolate from South korea identifies potential therapeutic options for COVID-19. Viruses 2021, 13, 651.
- Yadav, R.; Chaudhary, J.; Jain, N.; Chaudhary, P.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of structural and non-structural proteins and therapeutic targets of SARS-CoV-2 for COVID-19. Cells 2021, 10, 821.
- Finkelstein, M.T.; Mermelstein, A.G.; Parker Miller, E.; Seth, P.C.; Stancofski, E.D.; Fera, D. Structural analysis of neutralizing epitopes of the SARS-CoV-2 spike to guide therapy and vaccine design strategies. Viruses 2021, 13, 134.
- Lai, C.-C.; Chen, C.-H.; Wang, C.-Y.; Chen, K.-H.; Wang, Y.-H.; Hsueh, P.-R. Clinical efficacy and safety of remdesivir in patients with COVID-19: A systematic review and network meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 2021, 76, 1962–1968.
- Kocayiğit, H.; Süner, K.Ö.; Tomak, Y.; Demir, G.; Yaylacı, S.; Dheir, H.; Güçlü, E.; Erdem, A.F. Observational study of the effects of Favipiravir vs Lopinavir/Ritonavir on clinical outcomes in critically Ill patients with COVID-19. J. Clin. Pharm. Ther. 2021, 46, 454–459.
- Saw, P.E.; Song, E.-W. siRNA therapeutics: A clinical reality. Sci. China Life Sci. 2020, 63, 485–500.
- Zanella, I.; Zizioli, D.; Castelli, F.; Quiros-Roldan, E. Tenofovir, another inexpensive, well-known and widely available old drug repurposed for SARS-CoV-2 infection. Pharmaceuticals 2021, 14, 454.
- Dejmek, M.; Konkol’ová, E.; Eyer, L.; Straková, P.; Svoboda, P.; Šála, M.; Krejčová, K.; Růžek, D.; Boura, E.; Nencka, R. Non-Nucleotide RNA-Dependent RNA Polymerase Inhibitor That Blocks SARS-CoV-2 Replication. Viruses 2021, 13, 1585.
- Singh, D.D.; Han, I.; Choi, E.H.; Yadav, D.K. Immunopathology, host-virus genome interactions, and effective vaccine development in SARS-CoV-2. Comput. Struct. Biotechnol. J. 2020, 18, 3774–3787.
- Hurt, A.; Wheatley, A. Neutralizing antibody therapeutics for COVID-19. Viruses 2021, 13, 628.
- Li, X.; Zhang, L.; Chen, S.; Ouyang, H.; Ren, L. Possible targets of Pan-coronavirus antiviral strategies for emerging or re-emerging Coronaviruses. Microorganisms 2021, 9, 1479.
- Stoddard, S.V.; Wallace, F.E.; Stoddard, S.D.; Cheng, Q.; Acosta, D.; Barzani, S.; Bobay, M.; Briant, J.; Cisneros, C.; Feinstein, S.; et al. In silico design of peptide-based SARS-CoV-2 fusion inhibitors that target wt and mutant versions of SARS-CoV-2 HR1 Domains. Biophysica 2021, 1, 311–327.
- Yamamoto, M.; Kiso, M.; Sakai-Tagawa, Y.; Iwatsuki-Horimoto, K.; Imai, M.; Takeda, M.; Kinoshita, N.; Ohmagari, N.; Gohda, J.; Semba, K.; et al. The anticoagulant nafamostat potently inhibits SARS-CoV-2 s protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses 2020, 12, 629.
- Sardanelli, A.M.; Isgrò, C.; Palese, L.L. SARS-CoV-2 main protease active site ligands in the human metabolome. Molecules 2021, 26, 1409.
- Citarella, A.; Scala, A.; Piperno, A.; Micale, N. SARS-CoV-2 Mpro: A potential target for peptidomimetics and small-molecule inhibitors. Biomolecules 2021, 11, 607.
- Riva, A.; Conti, F.; Bernacchia, D.; Pezzati, L.; Sollima, S.; Merli, S.; Siano, M.; Lupo, A.; Rusconi, S.; Cattaneo, D.; et al. Da-runavir does not prevent SARS-CoV-2 infection in HIV patients. Pharmacol. Res. 2020, 157, 104826.
- Nersisyan, S.; Shkurnikov, M.; Turchinovich, A.; Knyazev, E.; Tonevitsky, A. Integrative analysis of miRNA and mRNA sequencing data reveals potential regulatory mechanisms of ACE2 and TMPRSS2. PLoS ONE 2020, 15, e0235987.
- Gatti, M.; Turrini, E.; Raschi, E.; Sestili, P.; Fimognari, C. Janus kinase inhibitors and Coronavirus dDisease (COVID)-19: Rationale, clinical evidence and safety issues. Pharmaceuticals 2021, 14, 738.
- Arisan, E.D.; Dart, A.; Grant, G.H.; Arisan, S.; Cuhadaroglu, S.; Lange, S.; Uysal-Onganer, P. The prediction of miRNAs in SARS-CoV-2 genomes: Hsa-miR databases identify 7 Key miRs linked to host responses and virus pathogenicity-related KEGG pathways significant for comorbidities. Viruses 2020, 12, 614.
- Chien, M.; Anderson, T.K.; Jockusch, S.; Tao, C.; Li, X.; Kumar, S.; Russo, J.J.; Kirchdoerfer, R.N.; Ju, J. Nucleotide Analogues as Inhibitors of SARS-CoV-2 Polymerase, a Key Drug Target for COVID-19. J. Proteome Res. 2020, 19, 4690–4697.
- Belouzard, S.; Millet, J.K.; Licitra, B.N.; Whittaker, G.R. Mechanisms of Coronavirus cell entry mediated by the viral spike protein. Viruses 2012, 4, 1011–1033.
- Gil Martínez, V.; Avedillo Salas, A.; Santander Ballestín, S. Antiviral therapeutic approaches for SARS-CoV-2 infection: A systematic review. Pharmaceuticals 2021, 14, 736.
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Saluk-Bijak, J.; Bijak, M. Existing drugs considered as promising in COVID-19 therapy. Int. J. Mol. Sci. 2021, 22, 5434.
- Malhani, A.A.; Enani, M.A.; Sharif-Askari, F.S.; Alghareeb, M.R.; Bin-Brikan, R.T.; AlShahrani, S.A.; Halwani, R.; Tleyjeh, I.M. Combination of (interferon beta-1b, lopinavir/ritonavir and ribavirin) versus favipiravir in hospitalized patients with non-critical COVID-19: A cohort study. PLoS ONE 2021, 16, e0252984.
- Jonsdottir, H.R.; Bielecki, M.; Siegrist, D.; Buehrer, T.W.; Züst, R.; Deuel, J.W. Titers of neutralizing antibodies against SARS-CoV-2 are independent of symptoms of non-severe COVID-19 in young adults. Viruses 2021, 13, 284.
- Magro, P.; Zanella, I.; Pescarolo, M.; Castelli, F.; Quiros-Roldan, E. Lopinavir/ritonavir: Repurposing an old drug for HIV infection in COVID-19 treatment. Biomed. J. 2021, 44, 43–53.
