Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Catherine Yang and Version 2 by Rita Xu.
The green peach aphid (Myzus persicae Sulzer), a major and harmful chili aphid usually managed using chemical pesticides, is responsible for massive annual agricultural losses. The efficacy of two protein elicitors, PeaT1 and PeBC1, to stimulate a defensive response against M. persicae in chili was studied in this study.
- PeaT1
- PeBC1
- M. persicae
- induced plant resistance against aphids
1. Introduction
Herbivores and plants have developed a complex interaction over the course of their respective evolutions. Plants are damaged by herbivores and thus modify their physical structures to cope with this damage; as such, plants have developed an economical defense mechanism to protect themselves against herbivores [1]. Herbivores’ development, colonization, feeding, survival, and oviposition are all affected by the structures and substances, which also attract natural adversaries and urge them to develop defence mechanisms [1][2]. Plants have established two primarily constitutive defensive mechanisms to deal successfully with this harm [1]. Plants use physically compromised barriers to resist colonization, such as cuticle trichomes, callose, cell walls, and suberin, but anti-biotic allelochemicals impact or stimulate pest production, fertility, and insect durability [3].
Aphids are phloem-feeding insects that spread plant viruses by syphoning off plant sap, resulting in major agricultural losses [4][5]. Aphid defence responses have been explored in a variety of aphid–plant settings. In green peach aphids M. persicae, Arabidopsis thaliana was shown to be less viable. Infested leaves with Sulzer [6]. Dietary effects were generated in chilli plants, and volatile organic compounds were released, resulting in a repellent effect against infested Bemisia tabaci [7]. Brevicoryne brassicae resistance reduced the survival rate and population growth parameters of immature Plutella xylostella in Brassica napus [8].
Jasmonic acid (JA), salicylic acid (SA), and ethylene stimulate the defensive response in plants (ET) [9]. In plants, salicylic acid (SA) and jasmonic acid (JA) are essential regulators of the induced defensive response [9][10]. The defense against sucking–piercing insects has been associated to SA, while the defense against chewing insects has been linked to JA [11]. ET regulates a variety of defense-related mechanisms in plants [12]. Danaus plexippus promotes JA pathway activation but inhibits SA acquisition in oleander aphids, Aphis nerii; JA has the opposite effect in Asclepias syriaca [12]. Plant responses to herbivory and necrotrophic disease infestations, according to current knowledge, activate the JA and SA defence pathways [10]. Similarly, some elicitors and eliciting components in plants can behave as resistant protein- and nucleotide-binding factors, resulting in aphid resistance [13]. Only a few prior studies have shown that JA and SA have a role in aphid response induction via increased expression of genes including PR-1, PR-2, CHIT1, LOX1, and PAL, all of which have been identified as responses induced by JA–SA after aphid feeding [14][15].
M. persicae Sulzer, a major harmful pest of cucumber, maize, barley, wheat, and beans in China, has a direct impact on crop productivity and quality due to its feeding behaviour. Plant defence responses are triggered by biotic and abiotic elicitors [16]. Elicitors are linked to a variety of diseases, including fungus, bacteria, viruses, and oomycetes. The most prevalent elicitors are proteins, glycoproteins, peptides, lipids, and oligosaccharides [17]. They are divided into two categories: race-specific groups that only elicit a defense response in host plants, and general defence groups that stimulate a defense response in both host and non-host plants [18]. Elicitors are bio factors or chemicals that plants use as signal molecules to promote systemic acquired resistance to diseases or herbivores by activating multiple defensive pathways [19][20]. Microbe-associated molecular patterns (MAMPs) and herbivore-associated molecular patterns (HAMPs) created by herbivorous insect pests are both examples of elicitors. The majority of HAMPs have been identified in pests that are lepidopterous, dipterous, or orthopterous [19]. Volicitin, for example, was discovered in beet armyworms (Spodoptera exigua) as the first herbivore-induced elicitor [21]. Elicitor proteins (MAMPs) from fungal (e.g., Pep-13 and endo-1,4-xylanases from Phytophthora and Trichoderma, respectively) and bacterial pathogens (e.g., flg22 from bacterial flagella) diseases have also been discovered [22][23]. These elicitors play an important role in crop protection because they can induce pest resistance, reduce pest fitness, and limit pest feeding. Elicitors are proteins, glycoproteins, and lipoproteins that activate signalling pathways, the hypersensitive response (HR), and reactive oxygen and nitrogen species (ROS and RNS) responses in plants to generate resistance to diseases and herbivore pests [20][24][25]. Reactive oxygen species (ROS) and nitric oxide (NO), both of which govern metabolic and transcriptional changes, are produced by physiological responses to common processes such as protein phosphorylation or plasma membrane protein activation [20]. Because of the rising demand for food safety, numerous protein elicitors have been investigated as potential pesticide substitutes [26][27][28][29].
PeaT1, broadly specific elicitor examined in Alternaria tenuissima; PeBC1 in Botrytis cinerea and is thought to promote plant resistance via the JA and SA pathways. It activates defense enzymes and strengthens cell walls while also stimulating the production of other defense-related genes [30][31]. Because of their minimal mammalian toxicity and excellent host specificity, entomopathogenic fungi are crucial in the biological control of insect pests [32]. Furthermore, these fungi have the ability to develop as entophytes within various plant parts [32][33]. They also produce systemic resistance in plants against biotic stressors such as phytoparasites, diseases, and nematodes [34], Furthermore, entomopathogenic fungi boost plant development [35], upsurge in the yield [36], and increase the nourishment of plant [37] and the growth of roots [38][39]. Abiotic stresses such as drought [30], iron chlorosis [40], and salinity stress [41] are also mitigated by these fungi. Fungi’s ecological functions have the ability to boost plant health and provide a new perspective on developing novel plant protection techniques [40]. Similarly, certain elicitor proteins from necrotrophic and biotrophic fungal infections have recently been identified, exhibiting induced tolerance to pathogens and herbivores. For example, in A. thaliana, the elicitor PeBC1, which was cloned from the necrotrophic fungus B. cinerea, generated disease resistance [42][43]. PeaT1 (GenBank: EF030819.1) is a type of general elicitor isolated from A. tenuissima. It activates systemic acquired resistance via the SA pathway in plants, resulting in cell wall strengthening and the upregulation of defense-related genes and activation of defense enzymes [44][45]. PeaT1 has been shown to promote growth and strengthen resistance to abiotic stresses in wheat and rice plants [46]. The aim of this study was to look into the activity and molecular mechanism of the B. cinerea-derived elicitor protein PeBC1 and the A. tenuissima-derived elicitor protein PeaT1 in the induction of green peach aphid resistance in chili plants. The impacts of PeaT1 and PeBC1 on M. persicae control, as well as the roles and mechanisms of PeaT1 and PeBC1 on M. persicae control, are investigated in this work to analyze the prospective influence of PeaT1 and PeBC1 on M. persicae. Trichomes were discovered on the leaf’s surface structure, thus prompting researchers to examine the contents of the JA and SA gene expressions from JA and SA. This research also includes information on PeaT1 and PeBC1 function, mechanism, and effects in the integrated management of the green peach aphid (M. persicae).
2. M. persicae Activity
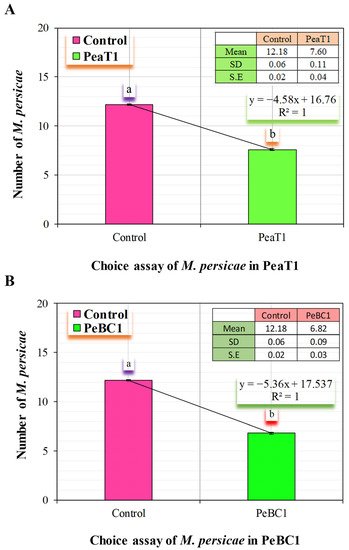
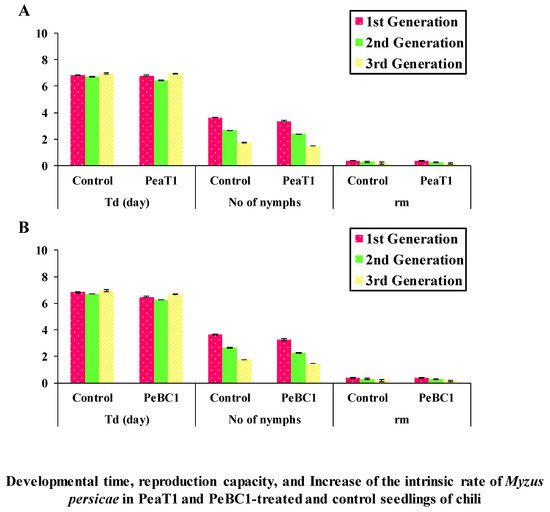
PeaT1 and PeBC1 used two separate strategies to generate resistance to M. persicae, the green peach aphid. First, aphid population fall was stable in PeaT1- and PeBC1-treated chili seedlings (Table 1 and Table 2), with percentage declines in population count in PeaT1 and PeBC1 treatments compared to the buffer and control treatments. M. persicae preferred to feed on the control chili seedlings in the host selection experiment. The frequency of M. persicae colonizing PeaT1- and PeBC1-treated plants was much lower a day after the aphid was inoculated and two days after spraying the seedlings than the control, which revealed aphid colonization in regions other than buffer and PeaT1- and PeBC1-treated areas. Some aphids chose to colonize control regions over those treated with PeaT1 and PeBC1 based on their feeding habits (Figure 1A,B). Second, aphids that were fed on seedlings treated with PeaT1 and PeBC1 had a longer developing period than those that were not, whereas M. persicae that were fed on seedlings treated with PeaT1 and PeBC1 had a lower everyday reproductive ability (second and third nymphal instars). The second and third generations grew at a slower pace (Figure 2A,B).
electrical penetration graph (EPG) data on PeBC1-treated and untreated chilli plants. Mean ± SD. Pathway activities are represented by C, potential drop is represented by Pd, phloem-feeding E represents activities, F represents penetration difficulty, G represents xylem-feeding activities, saliva injection is represented by E1, and sap sucking is represented by E2. Data were compared statistically using an independent t-test with two tails in SPSS 18.0. The difference between PeBC1 and control treatment with the same parameters of * (p = 0.05) is shown by asterisks.

Figure 1. M. persicae feeding preferences (A) M. persicae preferred feeding on control plants in PeaT1; (B) M. persicae feeding on PeBC1-treated and control-treated chilli seedlings 24 h after infestation colonization (mean ± SD). SPSS 18.0 was used to compare data using one-way analysis of variance (ANOVA) and the least significant difference (LSD). Lower style alphabet letters show significant variations across all treatments. (p = 0.05).

Figure 2. In PeaT1- and PeBC1-treated (A,B) and control seedlings of chili, developmental time, reproductive capacity, and growth of the intrinsic rate of M. persicae are reported as mean ± SD. Td stands for development period, nymphs per day stands for average reproduction ability, and rm stands for intrinsic rate rise. To compare data, SPSS 18.0 was utilized with one-way analysis of variance (ANOVA) and the least significant difference (LSD) (p = 0.05).
Table 1. M. persicae population variations were seen in PeaT1-, control-, and buffer-treated chili seedlings. To compare data, one-way analysis of variance (ANOVA), Levene’s test with SPSS 18.0, and the least significant difference (LSD) were employed. After aphid inoculation on the same day, significant variations in the letters in the rows can be noticed in all treated samples (p = 0.05).
| Days after Aphid Inoculation |
Control | Buffer | PeaT1 |
|---|---|---|---|
| 5 | |||
| 178.26 ± 0.02 | c |
Table 2. M. persicae population variations were seen in PeBC1-, control-, and buffer-treated chili seedlings. To compare data, one-way analysis of variance (ANOVA), Levene’s test with SPSS 18.0, and the least significant difference (LSD) were employed. After inoculation of aphid on the same day, significant changes in letters in rows can be noticed in all treated samples (p = 0.05).
| Days after Aphid Inoculation |
Control | Buffer | PeBC1 |
|---|
| EPG Parameters | Control ( | n = | 20) | PeBC1 ( | n = | 20) | |||
|---|---|---|---|---|---|---|---|---|---|
| 53.91 ± 0.05 | b | 58.24 ± 0.01 | a | 45.23 ± 0.02 | c | ||||
| 5 | 53.91 ± 0.05 | b | 57.40 ± 0.23 | a | 43.12 ± 0.03 | ||||
| Total probing time (h) | 3.14 ± 0.02 | c | 2.12 ± 0.01 * | ||||||
| 10 | 108.21 ± 0.04 | ||||||||
| Number of C | b | 124.26 ± 0.04 | |||||||
| 10 | a | 108.21 ± 0.04 | b | 86.24 ± 0.05 | c | ||||
| 15.78 ± 0.77 | 123.35 ± 0.03 | a | 84.57 ± 0.04 | c | 15 | 214.31 ± 0.06 | b | 249.17 ± 0.05 | a |
| 15 | 214.31 ± 0.06 | b | 248.15 ± 0.03 | a | 175.34 ± 0.06 | c |
3. Feeding Activity of M. persicae by EPG
The overall illustration of chili resistance variables was provided by an EPG. M. persicae feeding activity was considerably affected in seedlings treated with PeaT1 and PeBC1 (Table 3 and Table 4). The probing period, the length of C (pathway operation in all tissues), and the sum of M. persicae Pd (potential decrease in cell punctures) in the PeaT1- and PeBC1-treated chilli seedlings were significantly reduced, whereas the period of non-probe time before the first E (phloem-feeding activity) and the total duration of F (penetration problems) increased significantly. During the non-probing period, there was no electrical contact between the aphid stylet and the plant. The non-probing period before the first E was noticeably improved in the PeaT1 and PeBC1 treatments, implying a repellent or deterrent surface feature in the PeaT1 and PeBC1-treated chilli seedlings. C waves show intercellular type motion and may act as a mechanical plant barrier. The shorter the C waves (<3 min) detected, the greater the mechanical difficulty in seedlings treated with PeaT1 and PeBC1. Additionally, a decreased Pd number (cell puncture) was linked to aphid resistance in plants, which could be attributed to mechanical difficulties (the PeaT1- and PeBC1-treated chili seedlings in present study). Wave E1 indicated aphid saliva injection during phloem-feeding activities into sieve elements. In contrast, the E2 wave (sap sucking during phloem-feeding activities) showed phloem sap injection with concurrent salivation, which could have reflected a mesophyll or vascular resistance factor. In the sieve element, an extended E1 indicated more plugging or defense compounds. There was, however, no substantial difference between the control and PeaT1 and PeBC1 treatments in the E2 period, indicating no or low variability in phloem compounds to confer resistance to M. persicae. However, the period of the F wave in the PeaT1- and PeBC1-treated chili seedlings was higher, indicating that PeaT1 and PeBC1 induced an enhanced mechanical defense. The EPG results suggested that the resistance induced by PeaT1 and PeBC1 was mainly due to the modification of physical defenses.
Table 3. M. persicae electrical penetration graph (EPG) data on PeaT1-treated and untreated chilli plants. Mean ± SD. Pathway activities are represented by C, potential drop is represented by Pd, phloem-feeding E represents activities, F represents penetration difficulty, G represents xylem-feeding activities, saliva injection is represented by E1, and sap sucking is represented by E2. Data were compared statistically using an independent t-test with two tails in SPSS 18.0. The difference between PeaT1 and control treatment with the same parameters of * (p = 0.05) is shown by asterisks.
| EPG Parameters | Control ( | n = | 20) | PeaT1 ( | n = | 20) |
|---|---|---|---|---|---|---|
| Total probing time (h) | 3.78 ± 0.05 | 2.96 ± 0.01 | ||||
| Number of C | 16.45 ± 0.04 | 26.73 ± 0.04 * | ||||
| 25.66 ± 1.61 * | Number of short probes (C < 3 min) | 9.12 ± 0.07 | 24.12 ± 0.16 | |||
| Duration of non-probe period before the 1st E (h) | ||||||
| Number of short probes (C < 3 min) | 8.67 ± 0.81 | 23.43 ± 1.21 *3.92 ± 0.06 | 3.89 ± 0.06 * | |||
| Duration of non-probe period before the 1st E (h) | 3.76 ± 0.13 | 3.87 ± 0.03 | Number of pd | 72.87 ± 0.05 | 36.42 ± 0.07 | |
| Number of pd | 72.18 ± 0.05 | 35.15 ± 0.03 | Mean duration of Pd(s) | 8.14 ± 0.04 | 7.69 ± 0.09 | |
| Mean duration of Pd(s) | 7.71 ± 0.06 | 7.23 ± 0.15 | Number of E1 | 5.23 ± 0.05 | 4.42 ± 0.07 | |
| Number of E1 | 4.14 ± 0.02 | 3.45 ± 0.08 | Mean duration of E1(min) | 8.91 ± 0.04 | 10.12 ± 0.07 | |
| Mean duration of E1(min) | 8.79 ± 0.07 | 9.67 ± 0.08 * | Number of E2 | |||
| Number of E2 | 0.88 ± 0.07 | 0.73 ± 0.07 * | ||||
| 0.64 ± 0.05 | 0.54 ± 0.02 | Mean duration of E2 (h) | 29.96 ± 0.10 | 43.78 ± 0.05 | ||
| Mean duration of E2 (h) | 29.74 ± 0.02Number of G | 0.86 ± 0.06 | 0.79 ± 0.08 | |||
| 43.27 ± 0.04 | ||||||
| Number of G | 0.74 ± 0.06 | 0.68 ± 0.13 | Mean Duration of G (min) | 19.14 ± 0.04 | 14.67 ± 0.05 | |
| Mean Duration of G (min) | 18.23 ± 0.05 | Number of F | 5.24 ± 0.05 | 3.13 ± 0.06 | ||
| mean duration of F (min) | 22.24 ± 0.03 | 52.97 ± 0.04 |
Table 4. M. persicae
| 13.57 ± 0.01 |
| Number of F |
| 4.67 ± 0.06 |
| 2.43 ± 0.03 |
| mean duration of F (min) |
| 21.46 ± 0.02 |
| ± 0.03 |
References
- Zhao, L.Y.; Chen, J.L.; Cheng, D.F.; Sun, J.R.; Liu, Y.; Tian, Z. Biochemical and molecular characterizations of Sitobion avenae-induced wheat defense responses. Crop Prot. 2009, 28, 435–442.
- Walling, L.L. The myriad plant responses to herbivores. J. Plant Growth Regul. 2000, 19, 195–216.
- Ahuja, I.; Rohloff, J.; Bones, A.M. Defence mechanisms of brassicaceae: Implications for plant-insect interactions and potential for integrated pest management. Sustain. Agric. 2009, 2, 623–670.
- Girousse, C.; Moulia, B.; Silk, W.; Bonnemain, J.L. Aphid infestation causes different changes in carbon and nitrogen allocation in alfalfa stems as well as different inhibitions of longitudinal and radial expansion. Plant Physiol. 2005, 137, 1474–1484.
- Jakobs, R.; Schweiger, R.; Müller, C. Aphid infestation leads to plant part-specific changes in phloem sap chemistry, which may indicate niche construction. New Phytol. 2019, 221, 503–514.
- De Vos, M.; Jander, G. Myzus persicae (green peach aphid) salivary components induce defence responses in Arabidopsis thaliana. Plant Cell Environ. 2009, 32, 1548–1560.
- Saad, K.A.; Mohamad Roff, M.N.; Hallett, R.H.; Idris, A.B. Aphid-induced Defences in Chilli Affect Preferences of the Whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Sci. Rep. 2015, 5, 13697.
- Nouri-Ganbalani, G.; Borzoui, E.; Shahnavazi, M.; Nouri, A. Induction of resistance against Plutella xylostella (L.) (Lep.: Plutellidae) by jasmonic acid and mealy cabbage aphid feeding in Brassica napus L. Front. Physiol. 2018, 9, 859.
- Salzman, R.A.; Brady, J.A.; Finlayson, S.A.; Buchanan, C.D.; Summer, E.J.; Sun, F.; Klein, P.E.; Klein, R.R.; Pratt, L.H.; Cordonnier-Pratt, M.M.; et al. Transcriptional profiling of sorghum induced by methyl jasmonate, salicylic acid, and aminocyclopropane carboxylic acid reveals cooperative regulation and novel gene responses. Plant Physiol. 2005, 138, 352–368.
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270.
- Lazebnik, J.; Frago, E.; Dicke, M.; van Loon, J.J.A. Phytohormone Mediation of Interactions Between Herbivores and Plant Pathogens. J. Chem. Ecol. 2014, 40, 730–741.
- Ali, J.G.; Agrawal, A.A. Asymmetry of plant-mediated interactions between specialist aphids and caterpillars on two milkweeds. Funct. Ecol. 2014, 28, 1404–1412.
- Smith, C.M.; Boyko, E.V. The molecular bases of plant resistance and defense responses to aphid feeding: Current status. Entomol. Exp. Appl. 2007, 122, 1–16.
- Shoresh, M.; Yedidia, I.; Chet, I. Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 2005, 95, 76–84.
- Derbalah, A.; Elsharkawy, M.M.; Hamza, A.; El-Shaer, A. Resistance induction in cucumber and direct antifungal activity of zirconium oxide nanoparticles against Rhizoctonia solani. Pestic. Biochem. Physiol. 2019, 157, 230–236.
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333.
- Ellis, J.G.; Rafiqi, M.; Gan, P.; Chakrabarti, A.; Dodds, P.N. Recent progress in discovery and functional analysis of effector proteins of fungal and oomycete plant pathogens. Curr. Opin. Plant Biol. 2009, 12, 399–405.
- Montesano, M.; Brader, G.; Palva, E.T. Pathogen derived elicitors: Searching for receptors in plants. Mol. Plant Pathol. 2003, 4, 73–79.
- Maffei, M.E.; Arimura, G.I.; Mithöfer, A. Natural elicitors, effectors and modulators of plant responses. Nat. Prod. Rep. 2012, 29, 1288–1303.
- Garcia-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early signaling events induced by elicitors of plant defenses. Mol. Plant-Microbe Interact. 2006, 19, 711–724.
- Alborn, H.T.; Turlings, T.C.J.; Jones, T.H.; Stenhagen, G.; Loughrin, J.H.; Tumlinson, J.H. An elicitor of plant volatiles from beet armyworm oral secretion. Science 1997, 276, 945–949.
- Brunner, Â.; Rosahl, S.; Lee, J.; Rudd, J.J.; Geiler, C.; Scheel, D.; Nu, T. associated pattern from Phytophthora transglutaminases. EMBO J. 2002, 21, 6681–6688.
- Felix, G.; Duran, J.D.; Volko, S.; Boller, T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999, 18, 265–276.
- Chen, M.; Zhang, C.; Zi, Q.; Qiu, D.; Liu, W.; Zeng, H. A novel elicitor identified from Magnaporthe oryzae triggers defense responses in tobacco and rice. Plant Cell Rep. 2014, 33, 1865–1879.
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plantĝ€” pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548.
- Hael-Conrad, V.; Perato, S.M.; Arias, M.E.; Martínez-Zamora, M.G.; Di Peto, P.D.L.Á.; Martos, G.G.; Castagnaro, A.P.; Díaz-Ricci, J.C.; Chalfoun, N.R. The elicitor protein AsES induces a systemic acquired resistance response accompanied by systemic microbursts and micro-hypersensitive responses in Fragaria ananassa. Mol. Plant-Microbe Interact. 2018, 31, 46–60.
- Javed, K.; Javed, H.; Mukhtar, T.; Qiu, D. Pathogenicity of some entomopathogenic fungal strains to green peach aphid, Myzus persicae Sulzer (Homoptera: Aphididae). Egypt. J. Biol. Pest Control 2019, 29, 92.
- Javed, K.; Javed, H.; Mukhtar, T.; Qiu, D. Efficacy of beauveria bassiana and verticillium lecanii for the management of whitefly and aphid. Pakistan J. Agric. Sci. 2019, 56, 669–674.
- Javed, K.; Qiu, D. Protein Elicitor PeBL1 of Brevibacillus laterosporus Enhances Resistance Against Myzus persicae in Tomato. Pathogens 2020, 9, 57.
- Bayat, F.; Mirlohi, A.; Khodambashi, M. Effects of endophytic fungi on some drought tolerance mechanisms of tall fescue in a hydroponics culture. Russ. J. Plant Physiol. 2009, 56, 510–516.
- Wang, H.; Yang, X.; Guo, L.; Zeng, H.; Qiu, D. PeBL1, a novel protein elicitor from Brevibacillus laterosporus strain A60, activates defense responses and systemic resistance in Nicotiana benthamiana. Appl. Environ. Microbiol. 2015, 81, 2706–2716.
- Vega, F.E.; Meyling, N.V.; Luangsa-Ard, J.J.; Blackwell, M. Fungal Entomopathogens, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; ISBN 9780123849847.
- Verma, V.C.; Gange, A.C. Advances in Endophytic Research; Springer: Cham, Switzerland, 2014; pp. 1–454.
- Sánchez-Rodríguez, A.R.; Raya-Díaz, S.; Zamarreño, Á.M.; García-Mina, J.M.; del Campillo, M.C.; Quesada-Moraga, E. An endophytic Beauveria bassiana strain increases spike production in bread and durum wheat plants and effectively controls cotton leafworm (Spodoptera littoralis) larvae. Biol. Control 2018, 116, 90–102.
- Behie, S.W.; Zelisko, P.M.; Bidochka, M.J. Endophytic insect-parasitic fungi translocate nitrogen directly from insects to plants. Science 2012, 336, 1576–1577.
- Kabaluk, J.T.; Ericsson, J.D. Metarhizium anisopliae seed treatment increases yield of field corn when applied for wireworm control. Agron. J. 2007, 99, 1377–1381.
- Behie, S.W.; Bidochka, M.J. Ubiquity of insect-derived nitrogen transfer to plants by endophytic insect-pathogenic fungi: An additional branch of the soil nitrogen cycle. Appl. Environ. Microbiol. 2014, 80, 1553–1560.
- Sasan, R.K.; Bidochka, M.J. The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae) is also an endophyte that stimulates plant root development. Am. J. Bot. 2012, 99, 101–107.
- Liao, X.; O’Brien, T.R.; Fang, W.; St. Leger, R.J. The plant beneficial effects of Metarhizium species correlate with their association with roots. Appl. Microbiol. Biotechnol. 2014, 98, 7089–7096.
- Sánchez-Rodríguez, A.R.; Barrón, V.; Del Campillo, M.C.; Quesada-Moraga, E. The entomopathogenic fungus Metarhizium brunneum: A tool for alleviating Fe chlorosis. Plant Soil 2016, 406, 295–310.
- Waller, F.; Achatz, B.; Baltruschat, H.; Fodor, J.; Becker, K.; Fischer, M.; Heier, T.; Hückelhoven, R.; Neumann, C.; Von Wettstein, D.; et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc. Natl. Acad. Sci. USA 2005, 102, 13386–13391.
- Zhang, Y.; Yang, X.; Zeng, H.; Guo, L.; Yuan, J.; Qiu, D. Fungal elicitor protein PebC1 from Botrytis cinerea improves disease resistance in Arabidopsis thaliana. Biotechnol. Lett. 2014, 36, 1069–1078.
- Zhang, Y.; Yang, X.; Liu, Q.; Qiu, D.; Zhang, Y.; Zeng, H.; Yuan, J.; Mao, J. Purification of novel protein elicitor from Botrytis cinerea that induces disease resistance and drought tolerance in plants. Microbiol. Res. 2010, 165, 142–151.
- Zhang, W.; Yang, X.; Qiu, D.; Guo, L.; Zeng, H.; Mao, J.; Gao, Q. PeaT1-induced systemic acquired resistance in tobacco follows salicylic acid-dependent pathway. Mol. Biol. Rep. 2011, 38, 2549–2556.
- Zhao, M.Z.; Yang, X.F.; Zhang, M.; Yuan, J.J.; Qiu, D.W. Purification and Bioactivities of a Protein from Alternaria tenuissima. Chin. J. Biol. Control. 2006, 23, 170–173.
- Shi, F.; Dong, Y.; Zhang, Y.; Yang, X.; Qiu, D. Overexpression of the peaT1 elicitor gene from alternaria tenuissima improves drought tolerance in rice plants via interaction with a myo-inositol oxygenase. Front. Plant Sci. 2017, 8, 970.
More
