You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 3 by Peter Tang and Version 2 by Amina Yu.
Profound bioactivities of raw breastmilk include benefits attributed to the dense and diverse natural microbiota, termed natural microbiota hereafter. Infants benefit from breastfeeding not just nutritionally, but by both ‘seeding and feeding’ the infant gut , providing microbes that seed the naïve gastrointestinal (GI or gut) ecosystem and nutritive components that feed both infant and microbial cells.
- breastmilk microbiota
- benefit–risk
- colonization resistance
1. Introduction
Many studies in the past decade have characterized both the natural microbiota of mammalian milks, including human breastmilk, and the benefits that the natural microbiota of milks provide to developing gut, immune, respiratory, and neural systems [1][2]. The ‘microbial seeding and feeding’ of the natural microbiota of milks in mammalian gut systems is now understood to contribute to ‘completeness’ of the natural microbiota of the gut essential to both stimulating balanced development of the immune system and providing ‘optimized colonization resistance’, suppression of enteropathogen growth and infection, with enhancement of clearance of enteropathogens, by the natural microbiota of healthy breastfed infants’ gastrointestinal systems [3].
Preterm and low birthweight infants appear to suffer higher risk of failure to thrive and morbidity and mortality from infections than full term infants, as discussed more fully in Section 3 and Section 4. One of the leading causes of infant mortality, severe inflammatory necrotizing enterocolitis (NEC), appears to be a disease of dysbiosis rather than associated with infection by a specific enteropathogen [4][5]. Thus, a uniquely vulnerable niche may exist in the dysbiotic gut ecosystem of preterm infants who lack a protective natural microbiota of the gut and may ingest pasteurized donor milk, lacking or depleted of the natural microbiota of the mother’s own milk or raw breastmilk from donors.
Formal methodologies for microbial risk assessment and benefit–risk assessment are ideal for developing evidence-based policies for pasteurizing human donor milk. However, no benefit–risk assessments or risk assessments for bacterial pathogens were identified that compared pasteurized and raw milks from humans. Regarding global risk management for donor breastmilk, Japan and Norway [6][7] choose to provide raw donor breastmilk to NICU infants. All human donor milk banks in Norway (See https://europeanmilkbanking.com/country/norway/, accessed on 29 December 2020) and some in Germany (See https://europeanmilkbanking.com/country/germany/, accessed on 29 December 2020) screen and provide raw donor breastmilk, as documented on the European Milk Bank Association website.
Fear and dread of microbes as germs that will kill us may factor strongly into a policy that is becoming more controversial with expanding knowledge of the natural microbiota of milks from -omics studies in this decade: the decision to require pasteurization of breastmilk from donors in most human milk banks around the world [8][9][10][11][12]. The fear of microbes as germs may entrench well-meaning scientists and regulators in misconceptions of 20th century science, and wall them off from full consideration of the tremendous advances in knowledge about the natural microbiota of milks, particularly the rich body of evidence for both benefits and risks of raw breastmilk. In addition, germophobia may be fueled by misinformation about formula milk to families around the world that discourages breastfeeding, despite the significant loss of benefits associated with infant formula compared to breastfeeding [13]. Regarding risk management for breastmilk and formula, a companion manuscript submitted to this special collection [14] addresses relevant issues of ‘managing our microbes’ and raises concerns about potential tradeoffs between economic motivations of the global infant formula industry and health benefits of raw breastmilk to infants and families.
No formal quantitative benefit–risk assessment or risk assessment was identified to date that assessed raw breastmilk and pasteurized donor breastmilk or the conditions when risks of pasteurized donor breastmilk might outweigh the benefits of raw breastmilk with its natural microbiota intact.
2. Might Scientific Revolutions Be Shifting Our Paradigms?
Dietert and Silbergeld [15] pointed out the need to insert the microbiota into our frameworks for assessing safety and risk to human superorganisms, holobionts of Homo sapiens, and microbial partners in health [16]. The evidence map for the breastmilk ecosystem generated herein (Figure 1) illustrates the major shifts in methods, concepts, and knowledge base [17] that microbiologist and physician Martin Blaser [18] described as the ‘microbiome revolution’. The advances of the ‘microbiome revolution’ are defining a ‘new normal science’ regarding the structures and functions of the dense and diverse microbes in our bodies and in milks as our partners in health, a new paradigm diverging from the 20th century paradigm of microbes as germs that could kill us.
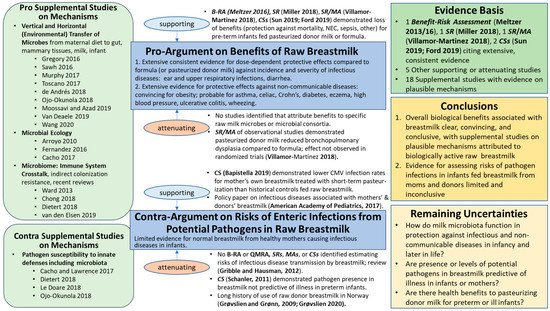

Figure 1. Evidence Map for Raw Breastmilk Ecosystem. B-RA = Benefit–Risk Assessment; QMRA = Quantitative Microbial Risk Assessment; CS = human cohort study; MA = meta-analysis; R = review; RT = randomized trial; SR = systematic review; and NEC = necrotizing enterocolitis. Referencing within this figure lists first author and date for those references cited in the text and subsequently with reference numbers: Meltzer et al., 2016 [19]; Meltzer et al., 2013 [20]; Miller et al., 2018 [21]; Villamor-Martinez et al., 2018 [22]; Sun et al., 2019 [23]; Ford et al., 2019 [24]; Bapistella et al., 2019 [25]; American Academy of Pediatrics, 2017 [8]; Gribble and Hausman, 2012 [26]; Schanler et al., 2011 [27]; Grøvslien and Grønn, 2009 [6]; Grøvslien, 2020 [28]. For Supplemental Studies on Mechanisms, first or first and second author(s) and year are listed within the figure, and full references provided in Supplementary Materials.
Consistent with Thomas Kuhn’s book, The Structure of Scientific Revolutions [29], this ‘change in paradigm’ (fundamental change in basic concepts and experimental practices, theories, models, or patterns of a scientific discipline) for raw breastmilk is supported by an extensive body of evidence on benefits and risks of human milk that, from our perspective, strongly challenges the validity of many outdated societal notions about interactions between the microbiota of milks and the host systems, particularly the immune system. For example, the notion that the presence of potential pathogens in raw milks certainly pose too high a health risk to permit even healthy infants, as well as pre-term or sick infants, to consume raw milks appears invalid. A paradigm shift may be necessary to incorporate advancing knowledge of milk ecosystems to develop policies that appropriately balance the benefits and risks of the raw milk microbiota for the 21st century.
Brief perspectives are offered below, highlighting key studies that illustrate the urgency for updating the ‘state of the science’ and uncertainties in order to begin new journeys on the difficult terrain of a shifting paradigm for considering the evidence for benefits and risks of pasteurizing donor breastmilk. Without a paradigm shift, development of evidence-based decision support for raw breastmilk and pasteurized donor milks seems impossible. Key updates are offered from 21st century perspectives of the natural microbiota of the breastmilk ecosystem, particularly microbiologic cross-talk with cells of innate and adaptive
2. Updating Earlier Notions from Science, Medicine, and Risk Analysis
The body of evidence for factors influencing the microbiota of milks related to the ‘environment’ aspect of the traditional ‘disease triangle’ (e.g., air quality and pollution; diet; supplements and pharmaceuticals; behavior/lifestyle/environment including farm and non-farm environments, built and natural environments, dust, soil; and water) is extensive and relevant to modeling dose–response relationships for pathogens amidst the natural microbiota of raw breastmilk and GI systems [30]. Some notable recent studies relevant for future dialogue with stakeholders include the following [31][32][33].
The 20th century notion that the microbiota of milks are simply contaminants posing high risk to human health appears invalid. Perceptions of bacteria as germs to be eradicated are gradually being replaced by deeper awareness of symbiotic (commensal and mutualistic) microbiota as our partners in health [3][16][34].
As humans are now recognized as a majority-microbial superorganism rather than simply a single-species type of mammal, analysis of benefits and risks needs to be directed toward the whole human, the superorganism. To enable this, old 20th century scientific dogmas concerning human health and safety as pertains to the immune system, microbes, human development, and safety evaluation must be discarded, and new paradigms established that align with 21st century science [34].
Dietert and Dietert [34] recently detailed seven 20th century dogmas that unduly affect science policy even though they are based on now-disproven science. The authors contend that these outdated scientific dogmas are impeding a progression toward much-needed sustainable healthcare. At least three of these outdated dogmas impact the consideration of benefit–risk for milk microbiomes: (1) the incorrect notion that the newborn’s immune system is completely balanced and fully functional at birth (significant immune maturation must happen in the infant to avoid predictable diseases); (2) the idea that all microbes are dangerous (most microbes are safe and many are needed); and (3) the idea that it is sufficient for safety assessment to focus on the human mammal (to the exclusion of safety for the human microbiome). This last outdated dogma resulted in existing approved drugs that present significant health risks for humans as they damage the human microbiome, such as proton pump inhibitors [34][35].
Knowledge about the microbiota of milks is contributing to a dramatic transformation of roles, not just of medical professionals, but also of parents and regulators, as ‘microbial managers’ of healthy microbiomes, to reduce susceptibility to or prevent disease and actively promote health [14]. This microbial management strategy is consistent with a need to shift emphasis from the epidemiologic disease triangle to a health triangle featuring the microbiota [30], as endorsed by others [36][37].
3. Updating Preconceived Notions on Breastmilk Ecosystem Structure and Function
Clearly, raw milks are not sterile [38][39][1][40][41][2], nor are the microbes present simply contaminants originating from feces or the environment. Rather, raw breastmilk is said to contain an “inimitable plethora of bioactive factors” that act “synergistically, making it difficult to delineate the specific functions of a given milk component” in isolation from the plethora of other factors present [42]. Despite substantial bodies of evidence linking beneficial effects to raw breastmilk (Figure 1; Supplemental Table S1), plausible mechanisms are incompletely characterized to date, largely due to the complexity of the functional networks of interactions within and between natural microbiota and other bioactive components [25][43][44].
The body of evidence documented herein provides strong support for the milk microbiota as beneficial for offspring development and maturation of GI, immune, neural, and respiratory systems in offspring. Further, evidence supports multiple origins of the microbes present. Recent reviews cite studies providing evidence regarding potential origins (niches, sources) of the natural microbiota of milks [39][1][40][41][2]. Thorough discussions of the evidence for breastmilk microbiota were provided by Zimmerman and Curtis [40] and Boudry and colleagues [2], and Oikonomou and colleagues [1] considered evidence on origins of milk microbiota across mammals.
Multiple lines of evidence support the plausible transfer of microbes from the infant oral (bucchal) and the maternal skin microbiomes, as well as an entero-mammary pathway for transfer of microbes or their DNA from the maternal GI tract to mammary tissue and subsequently to milk and the oral cavity and GI tract of breastfeeding infants [40][2][45]. Zimmerman and Curtis [40] document transfer of these gut bacterial genera to breastmilk: Bacteroides, Bifidobacterium, Blautia, Clostridium, Collinsella, Cutibacterium, Enterococcus, Escherichia, Lactobacillus, Parabacteroides, Pediococcus, Staphylococcus, Streptococcus, and Veillonella. Consistent with Zimmerman and Curtis, additional reviews [46][47] conclude that the predominance of available scientific evidence supports the entero-mammary pathway of transferring maternal GI microbes to breastmilk and breastfeeding infants. In multiple studies, probiotic strains administered during pregnancy were detected in the breastmilk ecosystem [40]. The review by Oikonomou and colleagues [1] cites some of this evidence, and concludes that the body of evidence suggests transfer of microbes from milk to infants via an entero-mammary route, though mechanistic details are not fully understood [2].
4. Including Benefits of Microbiota-Mediated Colonization Resistance
Given the natural microbiota of milks described above, competition within and between microbes in breastmilk is likely, and networks of microbes are linked by differential functional activities by direct and indirect competitive and cooperative relationships [43]. Similarly, direct and indirect microbial competition of the breastmilk microbiota with potential enteropathogens provide a primary disease prevention strategy with opportunities for more holistic, ecological approaches to ‘optimized colonization resistance’ in neonatology [3]. The importance of including evidence on dose- and time-dependent colonization resistance in microbial benefit and risk assessments was emphasized in recent studies [14][30].
Extensive data are now available that characterize plausible mechanisms driving infectious and inflammatory disease, including colonization resistance by direct and indirect competition of the microbiota in foods and the gut (Figure 1; Table 1; Supplemental Table S1). Colonization resistance likely enhances the health of superorganisms by multiple mechanisms simultaneously, including: (i) outcompeting pathogens for resources in the intestinal lumen; (ii) reducing likelihood of pathogen attachment along mucosal surfaces of the gut; (iii) up-shifting pathogen load requirements for disease (enhancing innate resistance against low pathogen doses); (iv) strengthening mucosal barriers against pathogenesis; and (v) optimizing immune homeostasis, balancing inflammatory processes linked with allergies, asthma, and infectious disease [3][48].
Table 1. Plausible mechanisms for colonization resistance (derived from Kim et al. [48]; Dietert [3]).
| Direct Mechanisms of Microbiota-Medicated Colonization Resistance | Indirect Mechanisms of Microbiota-Medicated Colonization Resistance: |
|---|---|
Outcompete enteropathogens for:
|
|
Antagonize or kill enteropathogens by directly producing:
|
Application of next generation microbial ecology and combinations of in vitro, in vivo, and microcosm experiments may support evidence-based policies for donor breastmilk that more effectively establishes a healthy gut microbiota and restores colonization resistance against nosocomial [49][50] and food-borne pathogens [48][51][52] in NICU infants in the future.
5. Updating Earlier Notions from Decision Science
The assumption that pasteurized donor milks are more beneficial and less risky to NICU infants is not supported by definitive evidence, particularly from microbiologic and immunologic perspectives (Figure 1). One could argue that the body of evidence is consistent with decreased health benefits with pasteurization, with the recent exception of a study documenting reduced risk for short-term pasteurization and CMV rates [25]. For some clinical outcomes, pasteurization may actually increase health risks to infants as evidenced in multiple clinical studies [21][22][24][20][23].
For nearly 80 years, Norway has documented no adverse effects or extra risk associated with its policy of screening and delivering raw breastmilk with non-detectable pathogens to mothers who are unable to breastfeed their infants, and pathogen-positive donor milk is destroyed rather than pasteurized. Including the full body of evidence, both supporting and attenuating, is important to expand deliberations around the world. Further, future deliberations should include evidence for potential pathogens representing high risk to infant health that merit screening in donor milk, not only to protect infant health, but also to maximize benefits to infant health.
Decisions by human donor milk banks to pasteurize all donor milk suggest that implicit barriers are preventing these organizations from considering the 21st century evidence for the breastmilk microbiota that might inform future evidence-based policies that account for both benefits and risks. It is possible that one of these implicit barriers is belief in the 20th century paradigm of germ theory based on fear that presence of potential pathogens alone causes high risk. The needs for deliberation of the evidence and analysis of both benefits and risks are urgent to optimize benefits and risks to NICU infants.
At present, the loss of the well-established benefits for raw breastmilk for many significant endpoints in neonatology by pasteurization [21][22][24][20][23] appears inconsistent with the ‘state of the science’ and uncertainties for infant health, and pasteurization appears associated with increased risk to NICU infants around the world. International deliberation of the evidence documented in Figure 1 is essential to development of evidence-based policies. The more holistic 21st century perspectives of the complexity and resilience of raw milk ecosystems documented herein and healthy gut ecosystems [14] may well benefit human health into childhood and adulthood, and outweigh acute infectious disease risks to NICU infants.
The authors propose the evidence map for the breastmilk ecosystem (Figure 1) as a starting point for an international workshop on the benefits and risks of pasteurization. Raw breastmilk and other foods containing a natural microbiota appear to contribute to GI, immune, neural, and respiratory system health for infants and adults. The workshop could launch a series of exercises of an analytic-deliberative process [53][54] to build shared understanding between experts and stakeholders in support of evidence-based risk management decisions on donor milk and other foods.
References
- Oikonomou, G.; Addis, M.F.; Chassard, C.; Nader-Macias, M.E.F.; Grant, I.; Delbès, C.; Even, S. Milk Microbiota: What Are We Exactly Talking About? Front. Microbiol. 2020, 11, 60.
- Boudry, G.; Charton, E.; Le Huerou-Luron, I.; Ferret-Bernard, S.; Le Gall, S.; Even, S.; Blat, S. The Relationship Between Breast Milk Components and the Infant Gut Microbiota. Front. Nutr. 2021, 8, 629740.
- Dietert, R.R. A Focus on Microbiome Completeness and Optimized Colonization Resistance in Neonatology. NeoReviews 2018, 19, e78–e88.
- Nolan, L.S.; Rimer, J.M.; Good, M. The Role of Human Milk Oligosaccharides and Probiotics on the Neonatal Microbiome and Risk of Necrotizing Enterocolitis: A Narrative Review. Nutrients 2020, 12, 3052.
- Seghesio, E.; De Geyter, C.; Vandenplas, Y. Probiotics in the Prevention and Treatment of Necrotizing Enterocolitis. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 245–255.
- Grøvslien, A.H.; Grønn, M. Donor Milk Banking and Breastfeeding in Norway. J. Hum. Lact. 2009, 25, 206–210.
- Mizuno, K.; Sakurai, M.; Itabashi, K. Necessity of Human Milk Banking in Japan: Questionnaire Survey of Neonatologists. Pediatr. Int. 2015, 57, 639–644.
- American Academy of Pediatrics (AAP) Committee Donor Human Milk for the High-Risk Infant: Preparation, Safety, and Usage Options in the United States. Pediatrics 2017, 139, e20163440.
- Klotz, D.; Jansen, S.; Glanzmann, R.; Haiden, N.; Fuchs, H.; Gebauer, C. Donor Human Milk Programs in German, Austrian and Swiss Neonatal Units-Findings from an International Survey. BMC Pediatr. 2020, 20, 235.
- Picaud, J.C.; Buffin, R.; Gremmo-Feger, G.; Rigo, J.; Putet, G.; Casper, C.; Working group of the French Neonatal Society on fresh human milk use in preterm infants. Review Concludes That Specific Recommendations Are Needed to Harmonise the Provision of Fresh Mother’s Milk to Their Preterm Infants. Acta Paediatr. 2018, 107, 1145–1155.
- Moro, G.E.; Billeaud, C.; Rachel, B.; Calvo, J.; Cavallarin, L.; Christen, L.; Escuder-Vieco, D.; Gaya, A.; Lembo, D.; Wesolowska, A.; et al. Processing of Donor Human Milk: Update and Recommendations From the European Milk Bank Association (EMBA). Front. Pediatr. 2019, 7, 49.
- World Health Organization (WHO). Donor Human Milk for Low-Birth-Weight Infants. Available online: https://www.who.int/elena/titles/donormilk_infants/en/ (accessed on 9 July 2019).
- World Health Organization (WHO). WHO/UNICEF Statement on the 40th Anniversary of the International Code of Marketing Breastmilk Substitutes. Available online: https://www.who.int/news/item/21-05-2021-WHO-UNICEF-statement-on-the-40th-anniversary-of-the-international-code-of-marketing-breastmilk-substitutes (accessed on 28 June 2021).
- Coleman, M.E.; Dietert, R.R.; North, D.W.; Stephenson, M.M. Enhancing Human Superorganism Ecosystem Resilience by Holistically Managing Our Microbes. Appl. Microbiol. 2021. Under review.
- Dietert, R.R.; Silbergeld, E.K. Biomarkers for the 21st Century: Listening to the Microbiome. Toxicol. Sci. 2015, 144, 208–216.
- Dietert, R.R. The Human Superorganism: How the Microbiome is Revolutionizing the Pursuit of a Healthy Life; Dutton: New York, NY, USA, 2016.
- North, D.W. Uncertainties, precaution, and science: Focus on the state of knowledge and how it may change. Risk Anal. 2011, 31, 1526–1529.
- Blaser, M.J. The Microbiome Revolution. J. Clin. Investig. 2014, 124, 4162–4165.
- Meltzer, H.M.; Knutsen, H.K.; Løland, B.F.; Odland, J.Ø.; Skåre, J.U.; Torheim, L.E.; Brandtzæg, P. Benefit and Risk Assessment of Breastmilk for Infant Health in Norway. Eur. J. Nutr. Food Saf. 2016, 6, 101–110.
- Meltzer, H.M.; Brandtzæg, P.; Knutsen, H.K.; Løland, B.F.; Odland, J.Ø.; Skåre, J.U.; Torheim, L.E. Benefit and Risk Assessment of Breastmilk for Infant Health in Norway; Scientific Opinion by the Scientific Steering Committee of VKM: Nydalen, Norway, 2013.
- Miller, J.; Tonkin, E.; Damarell, R.A.; McPhee, A.J.; Suganuma, M.; Suganuma, H.; Middleton, P.F.; Makrides, M.; Collins, C.T. A Systematic Review and Meta-Analysis of Human Milk Feeding and Morbidity in Very Low Birth Weight Infants. Nutrients 2018, 10, 707.
- Villamor-Martínez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Kramer, B.W.; Villamor, E. Donor Human Milk Protects against Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 238.
- Sun, H.; Han, S.; Cheng, R.; Hei, M.; Kakulas, F.; Lee, S.K. Testing the feasibility and safety of feeding preterm infants fresh mother’s own milk in the NICU: A pilot study. Sci. Rep. 2019, 9, 941.
- Ford, S.L.; Lohmann, P.; Preidis, G.A.; Gordon, P.S.; O’Donnell, A.; Hagan, J.; Venkatachalam, A.; Balderas, M.; Luna, R.A.; Hair, A.B. Improved feeding tolerance and growth are linked to increased gut microbial community diversity in very-low-birth-weight infants fed mother’s own milk compared with donor breast milk. Am. J. Clin. Nutr. 2019, 109, 1088–1097.
- Ojo-Okunola, A.; Nicol, M.; Du Toit, E. Human Breast Milk Bacteriome in Health and Disease. Nutrients 2018, 10, 1643.
- Sozańska, B. Raw Cow’s Milk and Its Protective Effect on Allergies and Asthma. Nutrients 2019, 11, 469.
- Keim, S.A.; Hogan, J.S.; McNamara, K.A.; Gudimetla, V.; Dillon, C.E.; Kwiek, J.J.; Geraghty, S.R. Microbial Contamination of Human Milk Purchased Via the Internet. Pediatrics 2013, 132, e1227–e1235.
- Lindemann, P.C.; Foshaugen, I.; Lindemann, R. Characteristics of breast milk and serology of women donating breast milk to a milk bank. Arch. Dis. Child.-Fetal Neonatal Ed. 2004, 89, F440–F441.
- Kuhn, T.S. The Structure of Scientific Revolutions; Fiftieth Anniversary; University of Chicago Press: Chicago, IL, USA, 2012.
- Coleman, M.; Elkins, C.; Gutting, B.; Mongodin, E.; Solano-Aguilar, G.; Walls, I. Microbiota and Dose Response: Evolving Paradigm of Health Triangle. Risk Anal. 2018, 38, 2013–2028.
- O’Connor, G.T.; Lynch, S.V.; Bloomberg, G.R.; Kattan, M.; Wood, R.A.; Gergen, P.J.; Jaffee, K.F.; Calatroni, A.; Bacharier, L.B.; Beigelman, A.; et al. Early-life home environment and risk of asthma among inner-city children. J. Allergy Clin. Immunol. 2017, 141, 1468–1475.
- Parajuli, A.; Grönroos, M.; Siter, N.; Puhakka, R.; Vari, H.K.; Roslund, M.I.; Jumpponen, A.; Nurminen, N.; Laitinen, O.; Hyoty, H.; et al. Urbanization Reduces Transfer of Diverse Environmental Microbiota Indoors. Front. Microbiol. 2018, 9, 84.
- Haahtela, T. A biodiversity hypothesis. Allergy 2019, 74, 1445–1456.
- Dietert, R.R.; Dietert, J.M. Twentieth Century Dogmas Prevent Sustainable Healthcare. Am. J. Biomed. Sci. Res. 2021, 13, 409–417.
- Naito, Y.; Kashiwagi, K.; Takagi, T.; Andoh, A.; Inoue, R. Intestinal Dysbiosis Secondary to Proton-Pump Inhibitor Use. Digestion 2018, 97, 195–204.
- Collineau, L.; Boerlin, P.; Carson, C.A.; Chapman, B.; Fazil, A.; Hetman, B.; McEwen, S.A.; Parmley, E.J.; Reid-Smith, R.J.; Taboada, E.; et al. Integrating Whole-Genome Sequencing Data into Quantitative Risk Assessment of Foodborne Antimicrobial Resistance: A Review of Opportunities and Challenges. Front. Microbiol. 2019, 10, 1107.
- EFSA Panel on Biological Hazards (EFSA BIOHAZ Panel); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Lindqvist, R. Whole Genome Sequencing and Metagenomics for Outbreak Investigation, Source Attribution and Risk Assessment of Food-Borne Microorganisms. EFSA J. 2019, 17, 05898.
- Fitzstevens, J.L.; Smith, K.C.; Hagadorn, J.I.; Caimano, M.J.; Matson, A.P.; Brownell, E.A. Systematic Review of the Human Milk Microbiota. Nutr. Clin. Pract. 2016, 32, 354–364.
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, 1039.
- Zimmermann, P.; Curtis, N. Breast milk microbiota: A review of the factors that influence composition. J. Infect. 2020, 81, 17–47.
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of Human Milk Bioactives on Infants’ Gut and Immune Health. Front. Immunol. 2021, 12, 290.
- Fernández, L.; Ruiz, L.; Jara, J.; Orgaz, B.; Rodríguez, J. Strategies for the Preservation, Restoration and Modulation of the Human Milk Microbiota. Implications for Human Milk Banks and Neonatal Intensive Care Units. Front. Microbiol. 2018, 9, 2676.
- Toscano, M.; De Grandi, R.; Peroni, D.G.; Grossi, E.; Facchin, V.; Comberiati, P.; Drago, L. Impact of delivery mode on the colostrum microbiota composition. BMC Microbiol. 2017, 17, 205.
- Ruiz, L.; García-Carral, C.; Rodríguez, J. Unfolding the Human Milk Microbiome Landscape in the Omics Era. Front. Microbiol. 2019, 10, 1378.
- Wang, S.; Ryan, C.A.; Boyaval, P.; Dempsey, E.M.; Ross, R.; Stanton, C. Maternal Vertical Transmission Affecting Early-life Microbiota Development. Trends Microbiol. 2019, 28, 28–45.
- Van Daele, E.; Knol, J.; Belzer, C. Microbial transmission from mother to child: Improving infant intestinal microbiota development by identifying the obstacles. Crit. Rev. Microbiol. 2019, 45, 613–648.
- Wang, J.; Kalyan, S.; Steck, N.; Turner, L.; Harr, B.; Künzel, S.; Vallier, M.; Häsler, R.; Franke, A.; Oberg, H.-H.; et al. Analysis of intestinal microbiota in hybrid house mice reveals evolutionary divergence in a vertebrate hologenome. Nat. Commun. 2015, 6, 6440.
- Kim, S.; Covington, A.; Pamer, E.G. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol. Rev. 2017, 279, 90–105.
- Stein, R.R.; Bucci, V.; Toussaint, N.; Buffie, C.G.; Rätsch, G.; Pamer, E.G.; Sander, C.; Xavier, J.B. Ecological Modeling from Time-Series Inference: Insight into Dynamics and Stability of Intestinal Microbiota. PLoS Comput. Biol. 2013, 9, e1003388.
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nat. Cell Biol. 2014, 517, 205–208.
- Becattini, S.; Pamer, E.G. Multifaceted Defense against Listeria monocytogenes in the Gastro-Intestinal Lumen. Pathogens 2017, 7, 1.
- Baumgartner, M.; Cardozo, K.P.; Hall, A. Microbial community composition interacts with local abiotic conditions to drive colonization resistance in human gut microbiome samples. Proc. R. Soc. B 2021, 288, 20203106.
- National Research Council (NRC). Understanding Risk: Informing Decisions in a Democratic Society; National Academies Press: Washington, DC, USA, 1996.
- North, D.W. Risk Analysis, Decision analysis, causal analysis, and economics: A personal perspective from more than 40 years experience. Risk Anal. 2020, 40, 2178–2190.
More
