Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Shin-Hoo Park and Version 2 by Lindsay Dong.
In the last decade, the Korean Laparoendoscopic Gastrointestinal Surgery Study group performed important clinical trials and exerted various efforts to enhance the quality of scientific knowledge and surgical techniques in the field of gastric cancer surgery. Laparoscopic gastrectomy has shifted to a new era in Korea due to recent advances and innovations in technology.
- minimally invasive surgery
- laparoscopy
- robotic surgery
- navigation surgery
- image-guided surgery
- indocyanine green
- bariatric surgery
- metabolic
1. Introduction
Since its introduction by Japanese surgeons in the early 1990s, in the last 30 years, laparoscopic surgery has rapidly gained international acceptance for the treatment of gastric cancer (GC) [1][2][1,2]. The Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS)-01 trial demonstrated that laparoscopic distal gastrectomy provided patients with early gastric cancer (EGC), with better cosmetic effects and pain reduction than open surgery [3][4][5][6][7][3,4,5,6,7]. The KLASS 02 trial demonstrated that laparoscopic distal gastrectomy for patients with advanced gastric cancer (AGC) was associated with a lower complication rate, faster recovery, and less pain with equivalent oncologic outcomes than open surgery [8][9][8,9].
In the last decade, Korean laparoscopic surgeons have made consistent efforts to improve the technique of laparoscopic gastrectomy (LG). Indeed, LG has shifted to a new era in Korea due to the increase in surgeons’ experience and recent innovations in surgical instruments and technology. The clinical advantages of LG can be further enhanced by reducing the number of trocars or the length of the incision. Therefore, reduced-port laparoscopic gastrectomy (RPLG) and single-incision laparoscopic gastrectomy (SILG) are increasingly performed in Korea [10][11][12][13][14][15][16][17][18][19][20][10,11,12,13,14,15,16,17,18,19,20]. As the surgical robot system has the advantages of precise movement without tremor and articulation with more degrees of freedom, surgeons have tried to overcome unresolved issues of laparoscopic surgery by performing robotic gastrectomy (RG) [21][22][23][24][25][21,22,23,24,25]. In addition, fluorescent image-guided navigation technology has achieved satisfactory outcomes in nodal localization, function preservation, and oncologic quality improvement [26][27][28][29][30][26,27,28,29,30]. One of the most astonishing outcomes is that obese patients with GC and diabetes have improved glycemic control after surgery for GC. These findings resulted in the use of the terminology “oncometabolic surgery” by Korean surgeons [31].
2. Reduced-Port and Single-Incision Laparoscopic Gastrectomy
2.1. Concept
RPLG was developed to overcome the technical difficulties of SILG. SILG, a surgery that integrates various efforts of minimal invasiveness by decreasing abdominal trauma, was first performed by Omori et al. [32] in 2011. This approach offers excellent cosmetic results because the scar is almost hidden in the umbilicus. Therefore, laparoscopic surgery should be performed via a single umbilical incision using a specially designed multichannel port. While conventional five-port LG requires triangulation regarding visualizing the laparoscopic surgical field and maneuvering the operator’s hands, SILG has a single dimension of surgical instruments and can be technically demanding even for experienced surgeons. Therefore, restriction of the working field and interference of laparoscopic instruments are the main technical issues associated with SILG [33]. However, additional ports and other lifting devices can alleviate these issues. Three-port totally laparoscopic distal gastrectomy (TLDG) uses one umbilical trocar for the laparoscopic camera and two trocars for the operator’s hands. Three-port TLDG is also called “duet-TLDG,” which emphasizes the fact that it is performed by a surgeon and scopist alone [16]. Two-port (dual-port) TLDG with an umbilical multichannel port plus one additional trocar is another option for overcoming the difficulties of SILG. RPLG does not require specialized instruments such as flexible scope or curved forceps; it can be easily performed by laparoscopic surgeons who are familiar with conventional LGs [14][19][14,19].
2.2. Operative Procedures
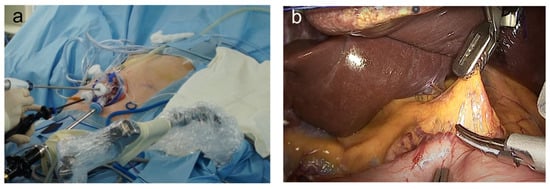
During reduced-port TLDG, the patient was placed in a reverse Trendelenburg supine position with the operator standing on the right side. Unlike some Japanese and Chinese surgeons who perform LG from the left side of the patient and then move to the right side, most Korean surgeons sat on the right side of the patient throughout the surgery. The scopist sat on the right side of, and caudal to, the patient. For R-duet TLDG, a 12 mm diameter trocar was inserted in the umbilical area (mainly for laparoscopy), and a 5 mm diameter trocar was placed in the right upper quadrant (RUQ) area, and 12 mm trocars in the right lower quadrant area [20]. For dual-port TLDG, a multichannel port (Gloveport, Nelis, Bucheon, Korea) was placed through the longitudinal 2.5–3 cm transumbilical incision. A 5 mm trocar was placed in the RUQ area [10]. During SILG, the patient was in a lithotomy supine position with reverse Trendelenburg. Meanwhile, the operator and scopist were positioned between the patient’s legs. A longitudinal 2.5 cm transumbilical skin incision was made. A commercial four-hole single port (Gloveport, Nelis) was then placed in the umbilical incision, and the abdominal cavity was insufflated with carbon dioxide at a pressure of 13 mmHg; no additional assistant trocar was used. A 10 mm flexible high-definition scope (Endoeye flexible HD camera system; Olympus Medical Systems Corp., Tokyo, Japan) and a 45 cm Harmonic scalpel (Ethicon Endo-Surgery Inc., Raritan, NJ, USA) were used to visualize every corner of the operative field and to facilitate dissection (Figure 1a) [11][12][11,12].
Figure 1. The current issues of single-incision laparoscopic gastrectomy in Korea: (a) outside view of single-incision laparoscopic gastrectomy; (b) laparoscopic view of single-incision laparoscopic gastrectomy.
2.3. Technical Feasibility and Surgical Outcomes
2.3. Technical Feasibility and Surgical Outcomes
Many Korean studies have demonstrated the technical feasibility and comparable surgical outcomes of three-port TLDG compared with conventional LG [14][15][16][18][20][14,15,16,18,20]. In addition, some authors have reported that three-port (R-duet) TLDG also allows high-quality lymph node (LN) dissection in patients with EGC [14][16][14,16]. However, to date, in Korea, the only improved surgical outcomes that have been reported include the following: less operative pain and scarring, reduced medical costs, and requiring fewer assistants. Regarding dual-port LG, enhanced minimal invasiveness has been reported in Japan and China. The dual-port TLDG was reported to have a lower postoperative complication rate than the conventional five-port TLDG [34][35]. Kawamura et al. reported that the amount of oral intake during the early postoperative period after dual-port TLDG exceeds that following conventional TLDG [35][36]. Shorter hospital stays and lower serum CRP levels were also demonstrated in dual-port TLDG [36][37]. Recently, a Korean single-arm study compared the surgical outcomes of dual port with those of dual-port TLDG and revealed that there were no significant differences in hospital stays, postoperative morbidities, operative time, time to first flatus, and diet between the two groups [10]. Korean and Japanese SILG case-matching studies analyzing the initial cases of pure single-port laparoscopic distal gastrectomy (SILDG) reported that it is both safe and feasible for patients with GC, with similar operation times and better short-term outcomes than conventional five-port TLDG including shorter hospital stays, earlier initiation of oral intake [37][38], less postoperative pain, less estimated blood loss, less inflammatory reaction, and improved cosmetic results [11][37][11,38].2.4. Oncologic Validity
To date, the long-term oncologic validity of reduced-port TLDG has rarely been investigated. A Korean retrospective multi-institutional study analyzed 1117 patients with GC and revealed that three-port and conventional five-port TLDG groups showed no significant differences in 5-year overall survival (94.3% vs. 96.7%, p = 0.138) or disease-free survival (94.3% vs. 95.9%, p = 0.231) [38][46].2.5. Controversial Issues and Future Perspectives
The application of RPLG or SILG in GC treatment remains controversial, and some surgeons have critically commented on these issues. LG should be conducted by experts and should never fail to exert the best of their abilities. Due to the possibility of interference and collision of surgical instruments, the best performance can be achieved by placing an additional port when necessary [39][48]. Moreover, the fourth edition of the Japanese gastric cancer treatment guidelines states that LG for GC treatment is an option for patients with stage I disease [40][34]. The KLASS-01, 02 trials demonstrated comparable long-term oncologic outcomes of laparoscopy-assisted distal gastrectomy, compared with open distal gastrectomy [4][5][9][4,5,9].3. Robotic Gastrectomy
3.1. Concept
Although the clinical advantages of LG have been demonstrated in terms of less postoperative pain, shorter hospital stays, and faster gastrointestinal function recovery, some problems remain unresolved, such as D2 lymph node dissection, anastomosis technique, and oncologic safety in AGC [8][9][8,9]. The da Vinci® Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) was introduced to overcome the drawbacks of laparoscopic surgery. Robotic surgical systems offer several advantages, including high-resolution 3D images, EndoWrist® with seven degrees of freedom, and tremor filtering. Thus, this technique is expected to increase the accuracy and thoroughness of minimally invasive gastrectomy [24][25][24,25].
3.2. Technical Feasibility and Surgical Outcomes
Some previous studies in Korea reported that RG had less intraoperative estimated blood loss than conventional LG [23][25][23,25]. This was in line with the results of previous studies with retrospective and systemic meta-analysis designs [41][42][58,59]. Park et al. [43][60] reported that reduced blood loss was more apparent in specific subgroups, such as patients with AGC who underwent D2 LN dissection or patients with a high body mass index (BMI).3.3. Operative Time and Learning Curve
A longer operative time has been reported in RG than in LG [41][42][44][58,59,66]. In Korea, a recent prospective multicenter study demonstrated that RG requires a longer operative time than LG [45][67]. Robotic surgery has inevitable time-consuming procedures, such as preoperative robot docking and preparation, changing of instruments performed by the assistant, and controlling the camera and instrument performed by the operator [46][68]. In addition, the surgeon that operates alone needs to perform the roles of assistant and camera scopist, which prolongs the operative time for RG [47][69]. Generally, surgeons are required to perform 20–25 cases to overcome the learning curve for RG, which is considerably less than the 60–90 cases required for LG [21][48][49][50][21,71,72,73]. Due to similarities in the operative procedure for RG and LG, performing sufficient laparoscopic procedures enable surgeons to easily perform RG.3.4. Oncologic Outcomes
Retrieving an adequate number of LN can offer accurate cancer staging with a more precise LN ratio system, thus improving survival in patients with GC [51][52][76,77]. LG with D2 LN dissection for AGC is a technically demanding procedure and is associated with high morbidity and mortality rates. Therefore, LG is typically considered a treatment option for patients with EGC. On the other hand, articulation with seven degrees of freedom and a comfortable environment with tremor elimination in robotic systems can enable surgeons to meticulously discern and finely dissect lymph nodes from surrounding complex lymphovascular structures or vital organs. Thus, RG is expected to be helpful in technically challenging procedures [25]. LN dissection near the supra-pancreatic or splenic-hilar area is considered one of the most technically difficult procedures in LG. Indeed, recent comparative studies showed that RG harvested more LNs from the suprapancreatic area than laparoscopic surgery [53][78].3.5. Future Perspectives
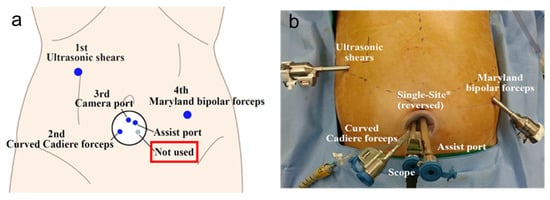
Recently, reduced port and single-incision surgery have been introduced in GC surgery, showing acceptable and feasible outcomes when performed by experienced surgeons [10][11][12][13][19][20][54][38][10,11,12,13,19,20,43,46]. These trends are also reflected in RG. In Korea, a phase I/II clinical trial demonstrated the safety and feasibility of reduced-port RG for EGC [55][85]. Seo et al. [56][86] introduced a modified technique using an infraumbilical single-site and two additional ports in the field of reduced-port totally robotic distal gastrectomy (Figure 2a,b). This novel technique resulted in acceptable surgical outcomes, with blood loss of 49.9 mL, operative time of 210 min, and the mean number of retrieved LNs of 58.8. The delta-shaped Billroth I reconstruction technique was applicable during reduced-port totally robotic distal gastrectomy using a similar approach with an infraumbilical single-site and two additional ports [57][87].
Figure 2. The current issues of robotic gastrectomy in Korea: (a) schematic illustrations of port placement of the single-site and two additional ports in robotic distal gastrectomy; (b) outside view of port placement of the single-site and two additional ports in robotic distal gastrectomy.
4. Fluorescence Image-Guided Gastrectomy
4.1. Concept
Resection of a sufficient number of lymph nodes (LNs) has become the current requirement for improved survival during GC surgery. In addition, it provides accurate staging using a precise LN ratio system [51][58][76,88]. However, radical laparoscopic lymphatic dissection is a technically demanding procedure due to complex vasculatures with multiple lymphatic channels surrounding the stomach. Careless handling and manipulation of the lymphovascular structures carry a high risk of tissue or vascular injury, which can lead to intra- or postoperative complications, such as fatal bleeding and pancreatic fistula [59][60][61][89,90,91]; however, it remains a substantial challenge, even for surgeons with a high level of proficiency. On the other hand, favorable and excellent outcomes obtained after treatment of patients with EGC have enabled the development of function-preserving gastrectomy with a focus on postoperative quality of life (QoL).
Image-guided surgery has been introduced in the field of surgical oncology due to recent advances in surgical technology. Indocyanine green (ICG) is a water-soluble tricarbocyanine dye that binds to albumin and has lower toxicity. Near-infrared (NIR) light has longer wavelengths and allows greater penetration into tissues. ICG-guided NIR imaging can provide better visualization of lymph nodes and lymphatic channels than other dying materials under visible light. Fluorescent image-guided surgery can help surgeons obtain additional anatomical information, such as identifying lymph nodes in thick fatty tissues, discerning lymphatic channels from vasculatures, identifying the origin and shape of a vessel, and assessing tissue perfusion status [26][62][63][64][65][66][26,93,94,95,96,97].
4.2. Oncologic Outcomes
As a new surgical navigation technique, ICG-guided NIR fluorescent imaging has shown improved oncologic quality in laparoscopic or robotic GC surgery. A recent prospective RCT demonstrated that the ICG NIR tracer-guided LG group had a noticeably increased number of dissected LNs and reduced LN noncompliance, compared with the conventional LG group (49.6 ± 15.0 vs. 41.7 ± 10.2%, p < 0.001; 31.8% vs. 57.4%, p < 0.001) [67][98].
4.3. Sentinel-Node Navigation Surgery
The application of intraoperative SN biopsy is expected to reduce unnecessary radical lymphadenectomy and allow function-preserving gastrectomy. In Korea, a prospective phase II trial was conducted to confirm the safety and feasibility of SN-navigation LG. Patients with positive SNs underwent gastrectomy with radical lymph node dissection (SN-positive group), whereas those with negative SNs received only limited gastric resections without further lymphadenectomy. The 3-year OS and RFS rates for the SN-negative group were 97.7% (95% C: 94.7–100.0%) and 95.5% (95% CI: 91.3–99.9%), respectively [68][99].4.4. Technical Advantages
Based on a technical viewpoint, Park et al. [30] demonstrated that NIR fluorescence guidance can provide safe and fast infrapyloric lymphadenectomy in laparoscopic distal gastrectomy. The operative time for infrapyloric LN dissection was significantly shorter in the ICG group than in the non-ICG group (13.1 ± 5.8 vs. 18.7 ± 7.9 min; p = 0.001), and the incidence of bleeding during infrapyloric LN dissection was lower in the ICG group (20% vs. 68.3%, p < 0.001). Identification of the infrapyloric artery (IPA) type is essential for safe pylorus-preserving gastrectomy. By visualizing the blood vessels and flow vividly, real-time NIR fluorescence navigation identified the IPA type, with a prediction rate of 80% [26].4.5. Controversial Issues and Future Perspectives
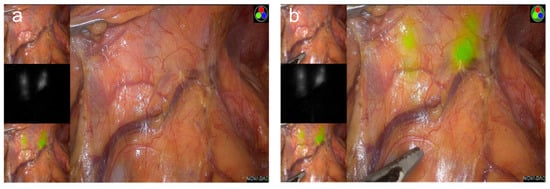
Better visualization of lymphatic channels, vessels, and LNs during GC surgery can encourage surgeons to achieve completeness of the lymphatic dissection without breakage of lymphatic structures. These detailed efforts can prevent tumor cell spillage and dissemination, ultimately resulting in improved oncologic quality. Moreover, complex, multiple lymphovascular structures within the stomach can become obstacles even for experienced surgeons. Fluorescence image guidance can help surgeons perform safer and even faster lymphatic dissection by preventing unexpected injuries when dissecting lymph node–bearing fatty tissue around blood vessels (Figure 3a,b).
Figure 3. The current issues of fluorescence image-guided gastrectomy in Korea: (a) laparoscopic view under visible light during laparoscopic distal gastrectomy; (b) indocyanine green-enhanced fluorescence uptake of lymphatic channels and lymph nodes during laparoscopic distal gastrectomy.
5. Oncometabolic Surgery
5.1. Concept
Originally, bariatric surgery was reported to cure morbid obesity; however, it was also highly effective in the treatment of chronic comorbidities of obese patients, such as type 2 diabetes mellitus (T2DM), dyslipidemia, and hypertension [69][109]. Among these, improvement of T2DM at 2–5 years postoperative has been particularly excellent, specifically 48.9–75.2% [70][71][72][73][110,111,112,113]. Bariatric surgery induced glycemic control independent of the resultant weight loss. These findings gave rise to the concept of “metabolic surgery” [13].
GC surgery and bariatric/metabolic surgery have similar operative procedures, including gastric resection and foregut bypass. Therefore, it can be hypothesized that GC surgery also has beneficial effects on patients’ glycemic control. Indeed, the improvement rate of T2DM after GC surgery was similar to that after bariatric/metabolic surgery [74][75][76][77][114,115,116,117]. These findings inspired the emergence of the terminology “oncometabolic surgery” [31], which targets the removal of malignancy and improved glycemic control with a one-step procedure. Considering that the incidence of T2DM is gradually increasing and that it is associated with increased mortality of patients with GC, oncometabolic surgery is expected to improve QoL and prolong the survival of patients with GC. However, a comparison of the baseline properties and operative procedures of the GC patient population and the obese patient population showed that these populations were not similar. Although the benefits of conventional GC surgery have already been confirmed regarding glycemic control, the degree of improvement may differ based on different operative procedures. Therefore, the procedures of oncometabolic surgery can be modified, carefully assessed, and engineered to maximize clinical benefit without compromising oncologic safety.
5.2. Patient Selection
Compared with bariatric populations, the patients who are candidates for oncometabolic surgery are typically older, have lower BMI, and present different pathophysiology for T2DM. The most notable difference between bariatric and oncometabolic populations is weight, and the degree of weight loss is an important contributing factor for improved glycemic control in bariatric surgery. Lee et al. [78][79][118,119] included the preoperative BMI in the ABCD score system, which aimed to predict the likelihood of improvement in glycemic control after metabolic surgery. Oncometabolic surgery should be reconsidered for patients with GC with a lower BMI who have difficulty achieving metabolic benefits from weight loss. Several Korean studies analyzing nonobese patients with GC investigated the impact of GC surgery on diabetes remission and demonstrated that surgery improved glycemic control in these patients [18][35][80][18,36,39].
5.3. Efficacy
In Korea, consistent efforts have been made to investigate the efficacy of GC surgery in the improvement of T2DM. However, studies reporting the efficacy of cancer surgery for improvement of glycemic status employed their own criteria for diabetes improvement (e.g., a decrease in the number of diabetic medications or a decrease in fasting plasma glucose or HbA1c levels). When the remission rates were displayed based on the American Diabetes Association (ADA) definition [81][125], the rate of diabetes remission ranged from 1.0% to 72.8% in partial gastrectomy with BII reconstruction group and from 27.3% to 90.5% in the TG with RY reconstruction group. Different follow-up periods and study designs may have resulted in wide variability in remission rates. Regardless of the employed criterion, TG with RY reconstruction is associated with the best efficacy in the improvement of glycemic control [31][75][79][82][83][84][31,115,119,120,122,126]. Generally, procedures with duodenal bypass (RY and BII reconstruction) showed better glycemic control than those without duodenal bypass (BI reconstruction) [75][79][85][83][115,119,121,122]. Weight-loss-induced metabolic effect on glycemic control may be minimal in nonobese patients with GC because patients with GC have a lower level of preoperative BMI than bariatric populations.5.4. Future Perspectives
T2DM is an important risk factor for mortality in patients with GC [86][130]. Improvement of diabetes following GC surgery is related to an increased 5-year survival rate [77][117]. These findings provide robust evidence that oncometabolic surgery may provide better survival outcomes in patients with GC with T2DM. Based on these findings, future studies are needed to investigate the impact of oncometabolic surgery on the survival of patients with GC.
6. Conclusions
GC surgery is still evolving through advances in surgical technology and devices, as well as the accumulation of knowledge and inspiration from older generations. However, given that long-term results of the described contents have not yet been verified, the current and updated surgical techniques and procedures should be carefully considered. Additionally, various efforts should be made to solve the issues of feasibility, training, education, and oncologic validity. Future prospective, well-designed multicenter studies are needed to provide reasonable and robust evidence for the current updated contents of GC surgery in Korea.
