Hydrodynamics-based gene delivery (HGD) is an efficient method for transfecting hepatocytes with plasmid DNA
in vivo
, but always associated with transient and non-tissue-specific expression of a gene of interest (GOI).
piggy
Bac (PB) transposon system enables chromosomal integration of GOIs, and as a result long-term expression of GOI is possible. In this study, we combined these two technologies to enable liver-specific expression of GOI for a long time. Mice are first subjected to HGD with a PB transposase expression plasmid and a PB transposon containing the GOIs placed downstream of the stopper sequence flanked by
loxP
. When the same mice are next subjected to HGD with a liver-directed Cre expression plasmid, Cre-mediated excision of
loxP
-flanked stopper sequence in the chromosomally integrated transposon and subsequent expression of GOIs occur. This Cre/
loxP
-based regulatable gene switching system together with combined used of HGD and PB will be useful for
in situ
manipulation of hepatocyte genome in non-transgenic animals.
- conditional cell ablation
- Cre/loxP
- diphtheria toxin-A chain
- EGFP
- gene switching
- hepatic disorder
- hydrodynamics-based gene delivery
- in vivo gene delivery
- liver
- piggyBac transposon
1. Hydrodynamics-Based Gene Delivery (HGD) Method
Hydrodynamics-based gene delivery (HGD) method
In animal experiment, tail-vein injection of nucleic acids (NAs) (as exemplified by plasmid DNA) is simple and convenient for NAs delivery into a living organism. HGD is performed by a hydrodynamic force generated by a pressurized injection of a large volume of DNA solution into the blood vessel. As a result, the force leads to generation of transient pores in the hepatocyte cell membrane. While the pores exist, NAs present in the solution enter the hepatocytes and are trapped in the cells; part of them migrates to the nucleus, where they facilitate targeted gene expression
. Unfortunately, gene expression in the liver is generally transient, because the NAs introduced by the HGD is often refractory to chromosomal integration into liver genome
[3]
. Furthermore, other organs (such as lung and kidney) besides the liver are often transfected, since it is mediated by tail-vein injection
[4]
.
2. piggyBac (PB) System Enabling Sustained Expression of a Gene of Interest (GOI)
Bac (PB) system enabling sustained expression of a gene of interest (GOI)
The PB system derived from the cabbage looper moth
Trichoplusia ni
[5]
is a one of the transposon–transposase system for efficient genetic modification of mammalian cells
[6]
. The PB transposase recognizes transposon-specific inverted terminal repeat sequences (ITRs) located on both ends of the transposon vector and efficiently integrates transgenes into the host genome
[7]
. However, the PB-mediated gene delivery system results in random integration of transgenes, leading to occasional transgene silencing, insertional mutagenesis, and positional variegation, probably as a result of transgene silencing
. The PB-based gene delivery has been reported to confer efficient chromosomal integration of GOIs in various types of
in vitro
cultured cell
, generation of transgenic (Tg) mice
[10]
, gene discovery
via
insertional mutagenesis
[12]
, and production of inducible pluripotent stem (iPS) cells
[13]
. Furthermore,
in vivo
long-term gene expression of GOI after HGD using PB-related vectors has been reported
[14]
.
3. Gene Switching System Enabling Conditional Liver-Specific Gene Expression of GOI
Gene switching system enabling conditional liver-specific gene expression of GOI
Although HGD is an efficient method enabling gene transfer into the liver more preferentially, it does not guarantee liver-specific gene expression. If a researcher wants to express a GOI only in the liver using HGD, the use of a liver-specific promoter for driving GOI expression is a promising approach. However, most liver-specific promoters identified to date have weaker promoter activity than the widely used universal promoter such as SV40 early or cytomegalovirus (CMV) promoter, which often makes it difficult to use liver-specific promoters to achieve stronger expression of a GOI.
There are several ways to enhance the activity of a tissue-specific but transcriptionally weak promoter
. For example, Nettelbeck et al.
[18]
provided a two-step amplification (TSTA) system, by which strong expression of GOI was induced by a strong promoter that had been activated through binding to the transcriptional activator produced from a weak promoter. We also established a similar two-step approach for enhancing the expression of a GOI by employing the Cre/
loxP
-based gene switching system
[19]
. By using this system, strong and liver-specific expression of a GOI could be successfully achieved even when a transcriptionally weak but liver-specific promoter was employed.
4. HGD with PB Transposons Confers Continuous Expression of GOI in Murine Liver
HGD with PB transposons confers continuous expression of GOI in murine liver
To examine whether PB transposons introduced into murine liver through HGD can guarantee the sustained expression of a GOI [enhanced green fluorescent protein (
EGFP
) cDNA in this case], mice were intravenously injected with a solution containing two PB-related vectors and a non-PB ptdTomato vector using HGD. The fluorescence of EGFP derived from PB vector was still detected on the liver samples isolated 56 days after HGD
[20]
. In contrast, the fluorescence of tdTomato derived from non-PB vector was undetectable on the samples isolated 28 days or more after HGD
[20]
. These results indicate that PB-based gene delivery is more effective for sustained gene expression of a GOI in murine liver.
5. Creation of Mouse Models for Hepatic Disorder Using Mouse Liver-Specific Gene Expression System
Creation of mouse models for hepatic disorder using mouse liver-specific gene expression system
To our knowledge, no reports of the both achievement of long-term and tissue-specific expression of a GOI
in vivo
have been published. To achieve this, we used our past established Cre/
loxP
-based gene switching system
[19]
, which employed both viral-derived universal promoter and liver-specific promoter.
Using this system, we attempted to express a protein of diphtheria toxin-A chain (DT-A) in mouse liver in a conditional manner. DT-A is known to be a potent protein leading to cell death through the inactivation of peptide elongation factor
[21]
. Mice were first subjected to HGD with a PB transposase expression plasmid and a PB transposon containing the GOIs (DT-A gene in this case) placed downstream of the stopper sequence flanked by
loxP
. When the same mice were next subjected to HGD with a liver-directed Cre expression plasmid (pTR/NCre), Cre-mediated excision of
loxP
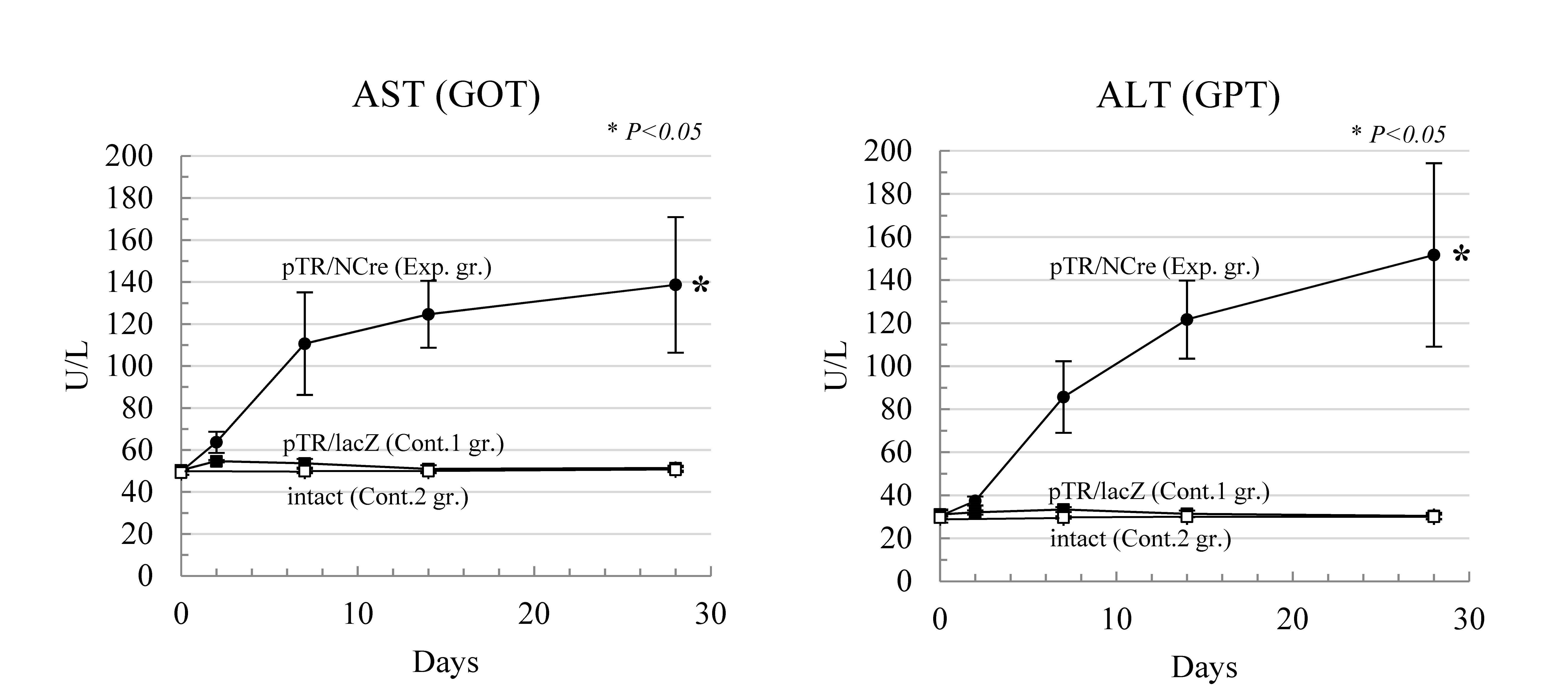
-flanked stopper sequence in the chromosomally integrated transposon and subsequent expression of DT-A gene occurred. Due to conditional expression of DT-A gene in mouse liver, hepatocyte death frequently occurred and as a result, liver disease was induced (Exp. gr. in Figure 1).
Figure 1.
HGD and PB transposon system enable the creation of disease models for liver dysfunction. When serum biochemistry profiles were assessed using blood samples, elevated levels of aspartate transaminase (AST) and alanine transaminase (ALT) in the experimental group were identified compared with those in control groups-1 (injection of pTR/LacZ) and -2 (intact). The level of significance set at
p
< 0.05 marked with an asterisk. Values are expressed as mean ± standard deviation among three mice tested for each group.
6. Conclusions
Conclusions
Using PB and HGD, we successfully achieved the sustained expression of a GOI in murine liver
[20]
. When Cre/
loxP
-based gene switching is combined with this PB-based HGD system, it is theoretically possible to induce liver-specific expression of GOI at any time the researcher wants. When we applied these technologies to produce liver disease model mice as proof-of-principle experiment, the resultant mice exhibited altered biochemical parameters and pathological abnormality. This present gene-based technology should also be useful for establishing an
in situ
manipulation system to assess liver function without the need to produce Tg animals.
References
- Mei Huang; Rui Sun; Qiang Huang; Zhigang Tian; Technical Improvement and Application of Hydrodynamic Gene Delivery in Study of Liver Diseases. Frontiers in Pharmacology 2017, 8, 591, 10.3389/fphar.2017.00591.
- Lei Zang; Makiya Nishikawa; Mitsuru Ando; Yuki Takahashi; Yoshinobu Takakura; Contribution of Epigenetic Modifications to the Decline in Transgene Expression from Plasmid DNA in Mouse Liver. Pharmaceutics 2015, 7, 199-212, 10.3390/pharmaceutics7030199.
- Takeshi Suda; Dexi Liu; Hydrodynamic Gene Delivery: Its Principles and Applications. Molecular Therapy 2007, 15, 2063-2069, 10.1038/sj.mt.6300314.
- F Liu; Y K Song; D Liu; Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Therapy 1999, 6, 1258-1266, 10.1038/sj.gt.3300947.
- Lynne Csiszar Cary; Michael Goebel; Bartholomew G. Corsaro; Hwei-Gene Wang; Elliot Rosen; M.J. Fraser; Transposon mutagenesis of baculoviruses: Analysis of Trichoplusia ni transposon IFP2 insertions within the FP-locus of nuclear polyhedrosis viruses. Virology 1989, 172, 156-169, 10.1016/0042-6822(89)90117-7.
- Fraser, M.J., Jr.; Carey, L.; Boonvisudhi, K.; Wang, H.G.H.; Assay for movement of Lepidepteran transposon IFP2 in insect cells using a Baculovirus genome as a target DNA.. Virology 1995, 211, 397-407, 10.1006/viro.1995.1422..
- M. J. Fraser; T. Clszczon; T. Elick; C. Bauser; Precise excision of TTAA-specific lepidopteran transposons piggyBac (IFP2) and tagalong (TFP3) from the baculovirus genome in cell lines from two species of Lepidoptera. Insect Molecular Biology 1996, 5, 141-151, 10.1111/j.1365-2583.1996.tb00048.x.
- Azahahianambi Palavesam; Caroline Esnault; David A. O’Brochta; Post-Integration Silencing of piggyBac Transposable Elements in Aedes aegypti. PLOS ONE 2013, 8, e68454, 10.1371/journal.pone.0068454.
- Valeri V. Mossine; James K. Waters; Mark Hannink; Thomas P. Mawhinney; piggyBac Transposon plus Insulators Overcome Epigenetic Silencing to Provide for Stable Signaling Pathway Reporter Cell Lines. PLoS ONE 2013, 8, e85494, 10.1371/journal.pone.0085494.
- Sheng Ding; Xiaohui Wu; Gang Li; Min Han; Yuan Zhuang; Tian Xu; Efficient Transposition of the piggyBac (PB) Transposon in Mammalian Cells and Mice. Cell 2005, 122, 473-483, 10.1016/j.cell.2005.07.013.
- Ding-Ping Bai; Ming-Ming Yang; Yulin Chen; PiggyBac Transposon-Mediated Gene Transfer in Cashmere Goat Fetal Fibroblast Cells. Bioscience, Biotechnology, and Biochemistry 2012, 76, 933-937, 10.1271/bbb.110939.
- Roland Rad; Lena Rad; Wei Wang; Juan Cadiñanos; George Vassiliou; Stephen Rice; Lia S. Campos; Kosuke Yusa; Ruby Banerjee; Meng Amy Li; et al.Jorge De La RosaAlexander StrongNg LuPeter EllisNathalie ConteFang Tang YangPentao LiuAllan Bradley PiggyBac Transposon Mutagenesis: A Tool for Cancer Gene Discovery in Mice. Science 2010, 330, 1104-1107, 10.1126/science.1193004.
- Knut Woltjen; Iacovos P. Michael; Paria Mohseni; Ridham Desai; Maria Mileikovsky; Riikka Hämäläinen; Rebecca Cowling; Wei Wang; Pentao Liu; Marina Gertsenstein; et al.Keisuke KajiHoon-Ki SungAndras Nagy piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 2009, 458, 766-770, 10.1038/nature07863.
- Hideyuki Nakanishi; Yuriko Higuchi; Shigeru Kawakami; Fumiyoshi Yamashita; Mitsuru Hashida; piggyBac Transposon-mediated Long-term Gene Expression in Mice. Molecular Therapy 2010, 18, 707-714, 10.1038/mt.2009.302.
- R. L. Brinster; J. M. Allen; R. R. Behringer; R. E. Gelinas; R. D. Palmiter; Introns increase transcriptional efficiency in transgenic mice.. Proceedings of the National Academy of Sciences 1988, 85, 836-840, 10.1073/pnas.85.3.836.
- M. Iyer; L. Wu; Michael Carey; Y. Wang; A. Smallwood; Sanjiv S. Gambhir; Two-step transcriptional amplification as a method for imaging reporter gene expression using weak promoters. Proceedings of the National Academy of Sciences 2001, 98, 14595-14600, 10.1073/pnas.251551098.
- Colin P.J. Glover; Alison S. Bienemann; Darren J. Heywood; A.Siobhan Cosgrave; James B. Uney; Adenoviral-Mediated, High-Level, Cell-Specific Transgene Expression: A SYN1-WPRE Cassette Mediates Increased Transgene Expression with No Loss of Neuron Specificity. Molecular Therapy 2002, 5, 509-516, 10.1006/mthe.2002.0588.
- D M Nettelbeck; V Jérôme; Rolf Müller; A strategy for enhancing the transcriptional activity of weak cell type-specific promoters. Gene Therapy 1998, 5, 1656-1664, 10.1038/sj.gt.3300778.
- Shingo Nakamura; Satoshi Watanabe; Masato Ohtsuka; Tadaaki Maehara; Masayuki Ishihara; Takaaki Yokomine; Masahiro Sato; Cre-loxP system as a versatile tool for conferring increased levels of tissue-specific gene expression from a weak promoter. Molecular Reproduction and Development 2008, 75, 1085-1093, 10.1002/mrd.20847.
- Shingo Nakamura; Masayuki Ishihara; Satoshi Watanabe; Naoko Ando; Masato Ohtsuka; Masahiro Sato; Intravenous Delivery of piggyBac Transposons as a Useful Tool for Liver-Specific Gene-Switching. International Journal of Molecular Sciences 2018, 19, 3452, 10.3390/ijms19113452.
- A M Pappenheimer, Jr; Diphtheria Toxin. Annual Review of Biochemistry 1977, 46, 69-94, 10.1007/978-1-4020-6754-9_4503.