Parkinson’s disease (PD) is a slowly progressive multisystem disorder affecting dopaminergic neurons of the substantia nigra pars compacta (SNpc), which is characterized by a decrease of dopamine (DA) in their striatal terminals. A crucial unmet demand in the management of Parkinson’s disease is the discovery of new approaches that could slow down, stop, or reverse the process of neurodegeneration. Novel potential treatments involving natural substances with neuroprotective activities are being developed. Curcumin is a polyphenolic compound isolated from the rhizomes of Curcuma longa (turmeric), and is considered a promising therapeutic and nutraceutical agent for the treatment of PD. However, molecular and cellular mechanisms that mediate the pharmacological actions of curcumin remain largely unknown. Stimulation of nicotinic receptors and, more precisely, selective α7 nicotinic acetylcholine receptors (α7-nAChR), have been found to play a major modulatory role in the immune system via the “cholinergic anti-inflammatory pathway”. Recently, α7-nAChR has been proposed to be a potential therapeutic approach in PD. In this review, the detailed mechanisms of the neuroprotective activities of curcumin as a potential therapeutic agent to help Parkinson’s patients are being discussed and elaborated on in detail.
- curcumin
- Parkinson’s disease
- antioxidant properties
- pharmacokinetics
1. Curcumin as a Potential Neuroprotective Agent
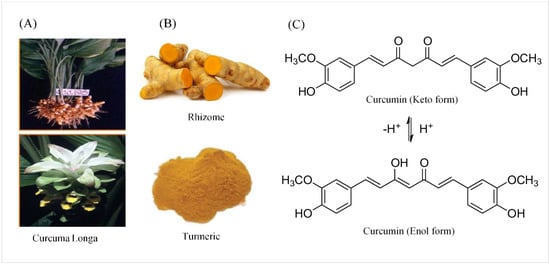
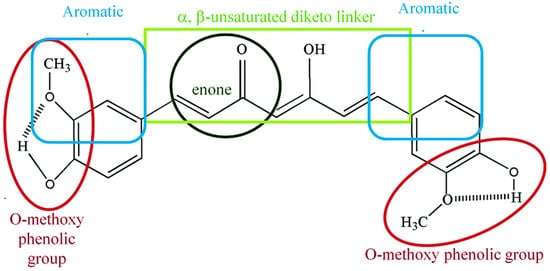
2. Chemical and Physical Properties of Curcumin
3. Pharmacokinetics and Pharmacodynamics of Curcumin
4. Biological Properties of Curcumin
5. Molecular and Cellular Neuroprotective Mechanisms of Curcumin in PD
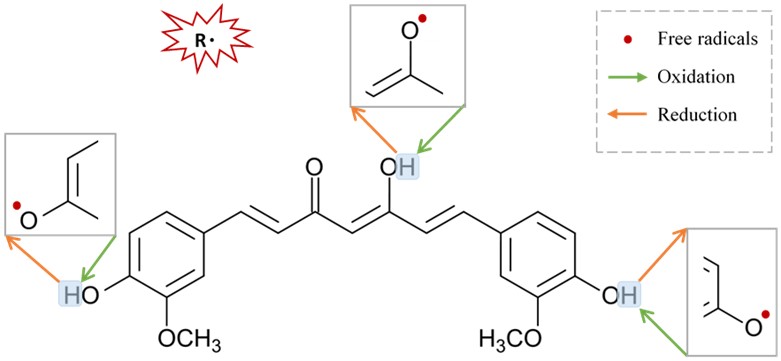 Figure 3. Suggested sites of exchange of phenol OH-group in curcumin structure with free radical oxidants, and its regeneration by a hydrogen donor antioxidant.
Figure 3. Suggested sites of exchange of phenol OH-group in curcumin structure with free radical oxidants, and its regeneration by a hydrogen donor antioxidant.67. Neuroprotective Mechanisms of Curcumin via Nicotinic Acetylcholine Receptors
Curcumin’s pharmacological actions are thought to be mediated by a variety of ligand-gated ion channels and receptors [66]. The recent study on the effects of the natural polyphenol compound provides evidence that curcumin possesses a potent neuroprotective effect as it preserves the integrity of the nigrostriatal dopaminergic system. This is distinctly manifested in the improved motor behavioral performance in the curcumin-treated animals through a α7-nAChRs-mediated mechanism [56]. This study adds to previous in vitro studies that show that curcumin enhances the effects of acetylcholine (ACh) through the function of α7-nAChRs in a concentration-dependent manner [67]. In addition, the results from another in vitro study highlight the significant role of curcumin in modulating the fluxes of calcium (Ca2+) ions via α7-nAChRs [68]. Based on the previous findings that curcumin acts as a type II PAM of α7-nAChRs and a potentiator of receptor function by significantly decreasing desensitization [67], it is reasonable to conclude that curcumin’s PAM action on α7-nAChRs has a beneficial effect in mediating neuroprotective effects [69][70]. Curcumin’s time-tested safety, neuroprotective efficacy, and preliminary clinical success of agents targeting nicotinic receptors in PD make it an appealing natural candidate for further investigation and development in the search for PD therapeutics. Our in vitro, in silico, and in vivo findings suggest that increasing Ca2+ influx may have a neuroprotective mechanism in neuronal and non-neuronal cells via various intracellular mechanisms, as shown in Figure 4 [56][67][68]. Stimulation of presynaptic α7-nAChR stimulates vesicular DA release via a Ca2+-dependent facilitation mechanism [71][72][73]. Extracellular signal-regulated mitogen-activated protein kinase (ERK/MAPK) activation can be triggered by protein kinase A (PKA) and/or calcium-calmodulin-dependent protein kinase (CaMK) [74]. A rise in intracellular Ca2+ levels is considered as a trigger factor of both signaling cascades. Activation of (ERK/MAPK) is a crucial signaling event in the cell survival pathway via upregulation of the cellular transcription factor; cAMP response element-binding (CREB), increasing gene expression of tyrosine hydroxylase and enhancing DA release [75][76]. α7-nAChR is also expressed on microglia and astrocytes and plays a major role in immune response via the “cholinergic anti-inflammatory pathway”. Activation of α7-nAChR results in an increase in intracellular Ca2+ concentration, and consequently modulates Janus kinase 2 (JAK2) and/or signal transducer and activator of transcription 3 (STAT3), ending up with an upregulation of protein kinase B (PKB), leading to inhibition of nuclear factor-kB (NFκB) [77]. The lipid signaling cascade that is started by protein kinase C (PKC), via phosphorylation of phosphatidylinositol 3-kinase (PI3K/Akt), is accredited with modulating the activities of neuroprotective and apoptotic factors, such as Bcl-2 and caspases, respectively [78][79][80]. Recent data demonstrate that the regulation of neuroinflammatory reactions by curcumin occurs through the modulation of the microglial JAK/STAT signaling pathway [81]. Collectively, all or some of these factors result in decreased apoptosis, enhance neuronal survival, modify immune responsiveness, and produce alteration in synaptic plasticity [82].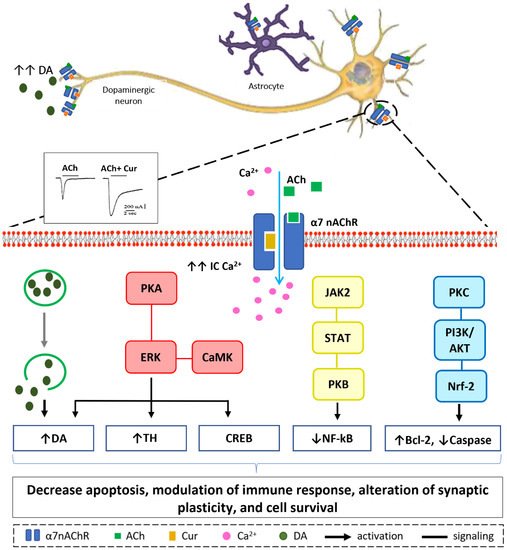
References
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299.
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2016, 7, 205–233.
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Mol. Basel Switz. 2020, 25, 1397.
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112.
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900.
- Wahlström, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. (Copenh.) 1978, 43, 86–92.
- Pandey, S.; Srivanitchapoom, P. Levodopa-induced Dyskinesia: Clinical Features, Pathophysiology, and Medical Management. Ann. Indian Acad. Neurol. 2017, 20, 190–198.
- Perkins, S.; Verschoyle, R.D.; Hill, K.; Parveen, I.; Threadgill, M.D.; Sharma, R.A.; Williams, M.L.; Steward, W.P.; Gescher, A.J. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2002, 11, 535–540.
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: The Golden Pigment from Golden Spice. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2014, 46, 2–18.
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147.
- Cheng, K.K.; Yeung, C.F.; Ho, S.W.; Chow, S.F.; Chow, A.H.L.; Baum, L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J. 2013, 15, 324–336.
- Ferrari, R.; Sponchioni, M.; Morbidelli, M.; Moscatelli, D. Polymer nanoparticles for the intravenous delivery of anticancer drugs: The checkpoints on the road from the synthesis to clinical translation. Nanoscale 2018, 10, 22701–22719.
- Shome, S.; Talukdar, A.D.; Choudhury, M.D.; Bhattacharya, M.K.; Upadhyaya, H. Curcumin as potential therapeutic natural product: A nanobiotechnological perspective. J. Pharm. Pharmacol. 2016, 68, 1481–1500.
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 487.
- Rachmawati, H.; Edityaningrum, C.A.; Mauludin, R. Molecular inclusion complex of curcumin-β-cyclodextrin nanoparticle to enhance curcumin skin permeability from hydrophilic matrix gel. AAPS PharmSciTech 2013, 14, 1303–1312.
- Siddique, Y.H.; Khan, W.; Singh, B.R.; Naqvi, A.H. Synthesis of alginate-curcumin nanocomposite and its protective role in transgenic Drosophila model of Parkinson’s disease. ISRN Pharmacol. 2013, 2013, 794582.
- Kundu, P.; Das, M.; Tripathy, K.; Sahoo, S.K. Delivery of Dual Drug Loaded Lipid Based Nanoparticles across the Blood-Brain Barrier Impart Enhanced Neuroprotection in a Rotenone Induced Mouse Model of Parkinson’s Disease. ACS Chem. Neurosci. 2016, 7, 1658–1670.
- Hahn, Y.-I.; Kim, S.-J.; Choi, B.-Y.; Cho, K.-C.; Bandu, R.; Kim, K.P.; Kim, D.-H.; Kim, W.; Park, J.S.; Han, B.W.; et al. Curcumin interacts directly with the Cysteine 259 residue of STAT3 and induces apoptosis in H-Ras transformed human mammary epithelial cells. Sci. Rep. 2018, 8, 6409.
- Zhang, N.; Yan, F.; Liang, X.; Wu, M.; Shen, Y.; Chen, M.; Xu, Y.; Zou, G.; Jiang, P.; Tang, C.; et al. Localized delivery of curcumin into brain with polysorbate 80-modified cerasomes by ultrasound-targeted microbubble destruction for improved Parkinson’s disease therapy. Theranostics 2018, 8, 2264–2277.
- White, C.M.; Pasupuleti, V.; Roman, Y.M.; Li, Y.; Hernandez, A.V. Oral turmeric/curcumin effects on inflammatory markers in chronic inflammatory diseases: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 146, 104280.
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience 2019, 406, 1–21.
- Burapan, S.; Kim, M.; Paisooksantivatana, Y.; Eser, B.E.; Han, J. Thai Curcuma Species: Antioxidant and Bioactive Compounds. Foods Basel Switz. 2020, 9, 1219.
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e2000171.
- Šudomová, M.; Hassan, S.T.S. Nutraceutical Curcumin with Promising Protection against Herpesvirus Infections and Their Associated Inflammation: Mechanisms and Pathways. Microorganisms 2021, 9, 292.
- Narayanan, V.S.; Muddaiah, S.; Shashidara, R.; Sudheendra, U.S.; Deepthi, N.C.; Samaranayake, L. Variable antifungal activity of curcumin against planktonic and biofilm phase of different candida species. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2020, 31, 145–148.
- Daily, J.W.; Yang, M.; Park, S. Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Med. Food 2016, 19, 717–729.
- Khan, H.; Ullah, H.; Nabavi, S.M. Mechanistic insights of hepatoprotective effects of curcumin: Therapeutic updates and future prospects. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 124, 182–191.
- Keihanian, F.; Saeidinia, A.; Bagheri, R.K.; Johnston, T.P.; Sahebkar, A. Curcumin, hemostasis, thrombosis, and coagulation. J. Cell. Physiol. 2018, 233, 4497–4511.
- Li, H.; Sureda, A.; Devkota, H.P.; Pittalà, V.; Barreca, D.; Silva, A.S.; Tewari, D.; Xu, S.; Nabavi, S.M. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol. Adv. 2020, 38, 107343.
- Den Hartogh, D.J.; Gabriel, A.; Tsiani, E. Antidiabetic Properties of Curcumin II: Evidence from In Vivo Studies. Nutrients 2019, 12, 58.
- Manarin, G.; Anderson, D.; Silva, J.M.E.; da Coppede, J.S.; Roxo-Junior, P.; Pereira, A.M.S.; Carmona, F. Curcuma longa L. ameliorates asthma control in children and adolescents: A randomized, double-blind, controlled trial. J. Ethnopharmacol. 2019, 238, 111882.
- Shahid, H.; Shahzad, M.; Shabbir, A.; Saghir, G. Immunomodulatory and Anti-Inflammatory Potential of Curcumin for the Treatment of Allergic Asthma: Effects on Expression Levels of Pro-inflammatory Cytokines and Aquaporins. Inflammation 2019, 42, 2037–2047.
- Fereydouni, N.; Darroudi, M.; Movaffagh, J.; Shahroodi, A.; Butler, A.E.; Ganjali, S.; Sahebkar, A. Curcumin nanofibers for the purpose of wound healing. J. Cell. Physiol. 2019, 234, 5537–5554.
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033.
- Saleh, M.M.; Darwish, Z.E.; El Nouaem, M.I.; Mourad, G.M.; Ramadan, O.R. Chemopreventive effect of green tea and curcumin in induced oral squamous cell carcinoma: An experimental study. Alex. Dent. J. 2020, 45, 74–80.
- Teng, C.-F.; Yu, C.-H.; Chang, H.-Y.; Hsieh, W.-C.; Wu, T.-H.; Lin, J.-H.; Wu, H.-C.; Jeng, L.-B.; Su, I.-J. Chemopreventive Effect of Phytosomal Curcumin on Hepatitis B Virus-Related Hepatocellular Carcinoma in A Transgenic Mouse Model. Sci. Rep. 2019, 9, 10338.
- Shishodia, S.; Singh, T.; Chaturvedi, M.M. Modulation of transcription factors by curcumin. Adv. Exp. Med. Biol. 2007, 595, 127–148.
- Golonko, A.; Lewandowska, H.; Świsłocka, R.; Jasińska, U.T.; Priebe, W.; Lewandowski, W. Curcumin as tyrosine kinase inhibitor in cancer treatment. Eur. J. Med. Chem. 2019, 181, 111512.
- Soung, Y.H.; Chung, J. Curcumin inhibition of the functional interaction between integrin α6β4 and the epidermal growth factor receptor. Mol. Cancer Ther. 2011, 10, 883–891.
- Palizgir, M.T.; Akhtari, M.; Mahmoudi, M.; Mostafaei, S.; Rezaiemanesh, A.; Shahram, F. Curcumin reduces the expression of interleukin 1β and the production of interleukin 6 and tumor necrosis factor alpha by M1 macrophages from patients with Behcet’s disease. Immunopharmacol. Immunotoxicol. 2018, 40, 297–302.
- Mhillaj, E.; Tarozzi, A.; Pruccoli, L.; Cuomo, V.; Trabace, L.; Mancuso, C. Curcumin and Heme Oxygenase: Neuroprotection and Beyond. Int. J. Mol. Sci. 2019, 20, 2419.
- Jin, H.; Qiao, F.; Wang, Y.; Xu, Y.; Shang, Y. Curcumin inhibits cell proliferation and induces apoptosis of human non-small cell lung cancer cells through the upregulation of miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol. Rep. 2015, 34.
- Hasima, N.; Aggarwal, B.B. Cancer-linked targets modulated by curcumin. Int. J. Biochem. Mol. Biol. 2012, 3, 328–351.
- Rainey, N.; Motte, L.; Aggarwal, B.B.; Petit, P.X. Curcumin hormesis mediates a cross-talk between autophagy and cell death. Cell Death Dis. 2015, 6, e2003.
- Sun, L.-R.; Zhou, W.; Zhang, H.-M.; Guo, Q.-S.; Yang, W.; Li, B.-J.; Sun, Z.-H.; Gao, S.-H.; Cui, R.-J. Modulation of Multiple Signaling Pathways of the Plant-Derived Natural Products in Cancer. Front. Oncol. 2019, 9, 1153.
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2016, 174, 1325–1348.
- Hassan, F.; Rehman, M.S.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front. Genet. 2019, 10, 514.
- Khatri, D.K.; Juvekar, A.R. Kinetics of Inhibition of Monoamine Oxidase Using Curcumin and Ellagic Acid. Pharmacogn. Mag. 2016, 12, S116–S120.
- Rabiei, Z.; Solati, K.; Amini-Khoei, H. Phytotherapy in treatment of Parkinson’s disease: A review. Pharm. Biol. 2019, 57, 355–362.
- Nguyen, T.T.; Vuu, M.D.; Huynh, M.A.; Yamaguchi, M.; Tran, L.T.; Dang, T.P.T. Curcumin Effectively Rescued Parkinson’s Disease-Like Phenotypes in a Novel Drosophila melanogaster Model with dUCH Knockdown. Oxid. Med. Cell. Longev. 2018, 2018, 2038267.
- Sharma, N.; Nehru, B. Curcumin affords neuroprotection and inhibits α-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology 2018, 26, 349–360.
- Di Martino, R.M.C.; Pruccoli, L.; Bisi, A.; Gobbi, S.; Rampa, A.; Martinez, A.; Pérez, C.; Martinez-Gonzalez, L.; Paglione, M.; Di Schiavi, E.; et al. Novel Curcumin-Diethyl Fumarate Hybrid as a Dualistic GSK-3β Inhibitor/Nrf2 Inducer for the Treatment of Parkinson’s Disease. ACS Chem. Neurosci. 2020, 11, 2728–2740.
- Ramires Júnior, O.V.; da Alves, B.S.; Barros, P.A.B.; Rodrigues, J.L.; Ferreira, S.P.; Monteiro, L.K.S.; de Araújo, G.M.S.; Fernandes, S.S.; Vaz, G.R.; Dora, C.L.; et al. Nanoemulsion Improves the Neuroprotective Effects of Curcumin in an Experimental Model of Parkinson’s Disease. Neurotox. Res. 2021, 39, 787–799.
- Abrahams, S.; Miller, H.C.; Lombard, C.; van der Westhuizen, F.H.; Bardien, S. Curcumin pre-treatment may protect against mitochondrial damage in LRRK2-mutant Parkinson’s disease and healthy control fibroblasts. Biochem. Biophys. Rep. 2021, 27, 101035.
- Motawi, T.K.; Sadik, N.A.H.; Hamed, M.A.; Ali, S.A.; Khalil, W.K.B.; Ahmed, Y.R. Potential therapeutic effects of antagonizing adenosine A2A receptor, curcumin and niacin in rotenone-induced Parkinson’s disease mice model. Mol. Cell. Biochem. 2020, 465, 89–102.
- El Nebrisi, E.; Javed, H.; Ojha, S.K.; Oz, M.; Shehab, S. Neuroprotective Effect of Curcumin on the Nigrostriatal Pathway in a 6-Hydroxydopmine-Induced Rat Model of Parkinson’s Disease is Mediated by α7-Nicotinic Receptors. Int. J. Mol. Sci. 2020, 21, 7329.
- Zbarsky, V.; Datla, K.P.; Parkar, S.; Rai, D.K.; Aruoma, O.I.; Dexter, D.T. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic. Res. 2005, 39, 1119–1125.
- Agrawal, S.S.; Gullaiya, S.; Dubey, V.; Singh, V.; Kumar, A.; Nagar, A.; Tiwari, P. Neurodegenerative Shielding by Curcumin and Its Derivatives on Brain Lesions Induced by 6-OHDA Model of Parkinson’s Disease in Albino Wistar Rats. Cardiovasc. Psychiatry Neurol. 2012, 2012, 942981.
- Yang, J.; Song, S.; Li, J.; Liang, T. Neuroprotective effect of curcumin on hippocampal injury in 6-OHDA-induced Parkinson’s disease rat. Pathol. Res. Pract. 2014, 210, 357–362.
- Velmurugan, B.K.; Rathinasamy, B.; Lohanathan, B.P.; Thiyagarajan, V.; Weng, C.-F. Neuroprotective Role of Phytochemicals. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 2485.
- Tirthraj, B.; Rohit, S. Role of Curcumin in Regulation of TNF-α Mediated Brain Inflammatory Responses. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 69–77.
- Alisi, I.O.; Uzairu, A.; Abechi, S.E.; Idris, S.O. Evaluation of the antioxidant properties of curcumin derivatives by genetic function algorithm. J. Adv. Res. 2018, 12, 47–54.
- Creţu, E.; Trifan, A.; Vasincu, A.; Miron, A. Plant-derived anticancer agents—Curcumin in cancer prevention and treatment. Rev. Med. Chir. Soc. Med. Nat. Iasi 2012, 116, 1223–1229.
- Treml, J.; Karel, Š. Flavonoids as Potent Scavengers of Hydroxyl Radicals: Flavonoids versus hydroxyl radical…. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738.
- Takahashi, R.; Ono, K.; Takamura, Y.; Mizuguchi, M.; Ikeda, T.; Nishijo, H.; Yamada, M. Phenolic compounds prevent the oligomerization of α-synuclein and reduce synaptic toxicity. J. Neurochem. 2015, 134, 943–955.
- Zhang, X.; Chen, Q.; Wang, Y.; Peng, W.; Cai, H. Effects of curcumin on ion channels and transporters. Front. Physiol. 2014, 5, 94.
- El Nebrisi, E.G.; Bagdas, D.; Toma, W.; Al Samri, H.; Brodzik, A.; Alkhlaif, Y.; Yang, K.-H.S.; Howarth, F.C.; Damaj, I.M.; Oz, M. Curcumin Acts as a Positive Allosteric Modulator of α7-Nicotinic Acetylcholine Receptors and Reverses Nociception in Mouse Models of Inflammatory Pain. J. Pharmacol. Exp. Ther. 2018, 365, 190–200.
- Nebrisi, E.E.; Al Kury, L.T.; Yang, K.-H.S.; Jayaprakash, P.; Howarth, F.C.; Kabbani, N.; Oz, M. Curcumin potentiates the function of human α7-nicotinic acetylcholine receptors expressed in SH-EP1 cells. Neurochem. Int. 2018, 114, 80–84.
- Corradi, J.; Bouzat, C. Understanding the Bases of Function and Modulation of α7 Nicotinic Receptors: Implications for Drug Discovery. Mol. Pharmacol. 2016, 90, 288–299.
- Uteshev, V.V. The therapeutic promise of positive allosteric modulation of nicotinic receptors. Eur. J. Pharmacol. 2014, 727, 181–185.
- Maex, R.; Grinevich, V.P.; Grinevich, V.; Budygin, E.; Bencherif, M.; Gutkin, B. Understanding the Role α7 Nicotinic Receptors Play in Dopamine Efflux in Nucleus Accumbens. ACS Chem. Neurosci. 2014, 5, 1032–1040.
- Cheng, Q.; Yakel, J.L. Presynaptic α7 Nicotinic Acetylcholine Receptors Enhance Hippocampal Mossy Fiber Glutamatergic Transmission via PKA Activation. J. Neurosci. 2014, 34, 124–133.
- Xu, Z.-Q.; Zhang, W.-J.; Su, D.-F.; Zhang, G.-Q.; Miao, C.-Y. Cellular responses and functions of α7 nicotinic acetylcholine receptor activation in the brain: A narrative review. Ann. Transl. Med. 2021, 9, 509.
- Kanasaki, H.; Purwana, I.; Oride, A.; Mijiddorj, T.; Miyazaki, K. Extracellular Signal-Regulated Kinase (ERK) Activation and Mitogen-Activated Protein Kinase Phosphatase 1 Induction by Pulsatile Gonadotropin-Releasing Hormone in Pituitary Gonadotrophs. J. Signal Transduct. 2011, 2012, e198527.
- Gu, X.; Liu, L.; Shen, Q.; Xing, D. Photoactivation of ERK/CREB/VMAT2 pathway attenuates MPP+-induced neuronal injury in a cellular model of Parkinson’s disease. Cell. Signal. 2017, 37, 103–114.
- Mohammadi, A.; Amooeian, V.G.; Rashidi, E. Dysfunction in Brain-Derived Neurotrophic Factor Signaling Pathway and Susceptibility to Schizophrenia, Parkinson’s and Alzheimer’s Diseases. Curr. Gene Ther. 2018, 18, 45–63.
- Zhu, R.-L.; Zhi, Y.-K.; Yi, L.; Luo, J.-F.; Li, J.; Bai, S.-S.; Liu, L.; Wang, P.-X.; Zhou, H.; Dong, Y. Sinomenine regulates CD14/TLR4, JAK2/STAT3 pathway and calcium signal via α7nAChR to inhibit inflammation in LPS-stimulated macrophages. Immunopharmacol. Immunotoxicol. 2019, 41, 172–177.
- Lin, Z.; Zhang, P.-W.; Zhu, X.; Melgari, J.-M.; Huff, R.; Spieldoch, R.L.; Uhl, G.R. Phosphatidylinositol 3-kinase, protein kinase C, and MEK1/2 kinase regulation of dopamine transporters (DAT) require N-terminal DAT phosphoacceptor sites. J. Biol. Chem. 2003, 278, 20162–20170.
- Zhou, H.; Li, X.-M.; Meinkoth, J.; Pittman, R.N. Akt Regulates Cell Survival and Apoptosis at a Postmitochondrial Level. J. Cell Biol. 2000, 151, 483–494.
- Long, H.-Z.; Cheng, Y.; Zhou, Z.-W.; Luo, H.-Y.; Wen, D.-D.; Gao, L.-C. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer’s Disease and Parkinson’s Disease. Front. Pharmacol. 2021, 12, 648636.
- Porro, C.; Cianciulli, A.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Curcumin Regulates Anti-Inflammatory Responses by JAK/STAT/SOCS Signaling Pathway in BV-2 Microglial Cells. Biology 2019, 8, 51.
- Shrestha, T.; Takahashi, T.; Li, C.; Matsumoto, M.; Maruyama, H. Nicotine-induced upregulation of miR-132-5p enhances cell survival in PC12 cells by targeting the anti-apoptotic protein Bcl-2. Neurol. Res. 2020, 42, 405–414.
