Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Muhammad Adeel and Version 2 by Rita Xu.
The ubiquitous presence of microplastics (MPs) and nanoplastics (NPs) in the environment is an undeniable and serious concern due to their higher persistence and extensive use in agricultural production.
- nanoplastics
- microbes
- rhizosphere
- Agricultural Plants
- Uptake and translocation
- Earthworms
- Fate and transport
- Toxicology
1. Introduction
Plastics are synthetic materials made up of polymers, which are long molecules around chains of carbons atoms, especially hydrogen, nitrogen, oxygen, and sulfur [1][2][1,2]. Plastics can be categorized based on their size, i.e., microplastics (>25 mm), mesoplastics (5–25 mm), microplastics (MPs) (0.1–5 mm), and nanoplastics (NPs) (<100 nm) [3]. Overall, worldwide plastic production is approximately 6300 million tons, of which 79% is deposited in landfills and other ecological segments [4]. In 2019, global plastic production was 368 million tons; 114.08 million tons of plastic were produced only in China and 58.88 million tons only in Europe, as shown in Figure 1, and its capacity is expected to double in 2040 [4][5][6][4,5,6].
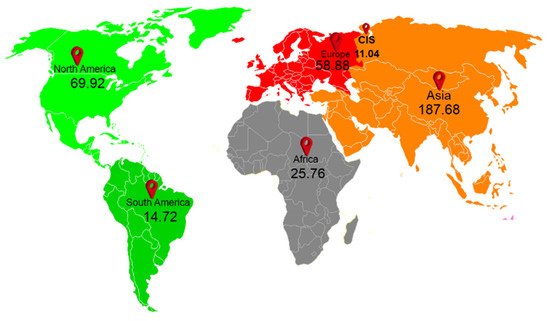
Figure 1. Worldwide production of plastics, numbers indicate in million tons. (The data is obtained from [6]).
Plastic is part of our daily lives; approximately 4 trillion plastic bags are used annually and 1 million plastic bottles are consumed every single minute [7]. These plastic bags or bottles are used for ~12 min and need years to decompose [8]. Unauthorized dumping and inadequate waste management lead to the release of environmental plastics and have a long environmental lifespan, which can easily accumulate in different environmental matrices [9]. MP contamination is a rapidly increasing concern throughout the world and has been listed as the second most emerging environmental and ecological issue [10][11][12][10,11,12] after global warming [13].
Soil, especially arable soil, has become a major and permanent sink for plastic, coming mostly from anthropogenic activities such as manufacturing {25.6–28% (Figure 2)} [4][14][15][16][17][4,14,15,16,17]. The extreme level of plastic pollution from wastewater treatment plants (WTPs) found on agricultural land is approximately 7.76 million tons [18]. In 2012, plastics being added to the soil through mulching by agricultural systems was equivalent to around 4.4 million tons [19][20][19,20]. Additionally, landfills sites also introduced plastics to the terrestrial ecosystem, but the exact data are not reported yet [21][22][21,22].
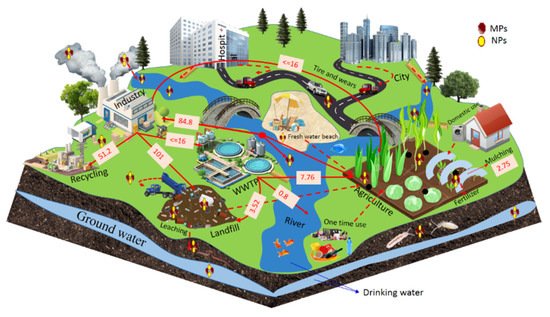
Figure 2. Estimates of plastic sources and transport pathways in the environment as reported in scientific literature; the data is indicated in million tons as well as the average data of high and low transportation in the ecosystem, arrows represent the known transportation and dashes represent unknown transportation in the ecosystem.
In territorial environments, MPs and NPs have varied toxic effects depending on the medium of exposure and interplay with other contaminants. Interactions with these pollutants can cause significant changes in the properties of plastic surfaces, and the agroecosystem can uptake these MPs and NPs and/or pollutants [23][24][25][23,24,25]. Co-exposure and the accumulation of these contaminants have been associated with antagonistic [26], synergistic [23][27][28][23,27,28], or additive effects [29]. Given the multifaceted nature of MPs and NPs, the study of contaminant interactions with MPs and NPs is a major concern in agroecosystem impact assessments. Raising chemicals, e.g., polybrominated diphenyl ether (PBDE), bisphenol A (BPA), and phthalates are commonly used to change the quality and performance of plastic. Nevertheless, plastics continue to deteriorate even after they are released into the ecosystem, and the use of these chemicals in synthetic plastic is more risky to animals, plants, and humans [30][31][32][33][34][35][36][30,31,32,33,34,35,36].
Agroecosystems are very prone to plastic contamination due to modern farming practices. Despite the potential entry of these emerging contaminants into the agricultural system, information on the impacts of MPs and NPs on soil biota, especially plants, is currently very scarce. As far as we know, only three studies have documented the effects of MPs and NPs on non-vascular plants [37][38][39][37,38,39] and only ten studies regarding the vascular plant have just been reported in the scientific literature [40][41][42][43][44][45][46][47][48][49][40,41,42,43,44,45,46,47,48,49]. Current literature detected the impact of MPs and NPs on bio-mass production and plant growth [49][50][51][52][53][49,50,51,52,53]. Detailed studies of NPs on wheat (Triticum aestivum) and broad beans (Vicia faba) have been published by Lian et al. [54] and Jiang et al. [48], respectively; moreover, two recent studies have investigated the effect of foliar application of NPs on the lettuce (Lactuca sativa) and maize (Zea mays) plants [46][47][46,47], which briefly discuss the toxic effects on plants.
2. Sources of Plastic in Agriculture Systems
Over the past six decades, plastics have become an essential and versatile product with a wide range of properties, chemical compositions, and applications. However, the release of plastic particles generated by the mass production of plastics is a major threat to the environment [55][56][55,56].
Plastic pollution, especially in the soil environment, adversely affects soil organisms and plants [57]. Plastic accumulates in the soil in different ways including through plastic packing, wastewater treatment plants, mulching, atmospheric deposition, and daily use products. The countless use of disposable plastic products cannot be separated from the serious pollution of MPs and NPs in soil [58].
In the market, the largest source of plastic comes from packaging [59] and with the rise of plastic resins, consumer marketplaces are getting more plastics day by day [60]. The use of plastic in packaging has rapidly increased and has made important contributions to facilitating daily life. Recent estimates show that more than 90 billion polyethylene bags are eliminated annually as non-renewable waste and trash [61]. The International Energy Agency (IEA) predicted that strong economic growth [62] will further increase the share of plastic packaging, i.e., 26% of the total volume in 2015, and could be doubled in the next 15 years with the increase of forecasted growth by up to four times by 2050 and up to 318 million tones/year, which is more than the entire current plastic industry [63]. Widespread use of plastics, known as “white pollution,” is becoming more serious in the environmental ecosystem [64]. All plastic that goes to landfills eventually reaches terrestrial/aquatic ecosystems.
The main sources of MPs and NPs in sewage wastewater treatments are fibers from clothing, microbeads from personal care products, cleaning products, and plastic debris [65][66][67][68][69][65,66,67,68,69]. A previous study determined that acrylic (17%) and polyester (67%) fibers were the main components of plastics in wastewater samples. Compared to actual waste material, it was found that the ratio was equivalent to the composition of textiles (5% acrylic and 78% polyester). Finally, it was concluded that sewage MPs come primarily from washing clothes [70]. In addition, through domestic washing machines, each wash can produce N1900 fibers. Usual wastewater treatment can release 300 million plastic debris into nearby watercourses every day [71]. Li et al. [72] showed that 90% of MPs accumulate in wastewater sludge and that its concentration in sludge ranges from 1500 to 56,400 particles kg−1 [73]. Most obviously, sludge is applied as fertilizer on agricultural land [69]. Similarly, another study documented that organic fertilizer has up to 895 particles kg−1 of MPs [74]. Hence, the continuous application of organic fertilizers and sludge can cause soil contamination with MPs [75][76][75,76], upsurge the contamination of extreme levels of farmlands, and are also harmful to plant and human health. The formation of a separate drainage system is of great importance for the immediate promotion of improved sludge treatment and handling services in agricultural systems.
In many countries, plastic mulching has become a widely adopted practice in agriculture for its immediate economic benefits [77]. On agricultural land, plastics, especially low-density polythene (LDPE), are used for mulching to enhance fruit and vegetable production. Undoubtedly, plastic mulching gives short-term benefits to growers by improving water-use efficiency in semi-arid regions, controlling some weeds, and earlier and late maturity of crops [78]. On the other hand, most of the world’s productive and valuable soil resources are exposed to plastic residue. For example, in China, about 20 million hectares of agricultural land are being exposed to mulching by plastics and is expected to increase by 7.1% or more per year [64][78][64,78]. In 2015, 1.455 million tons of plastic were used for mulching in China [79].
Once plastic accumulates in soil, it is technically very difficult to recycle or remove from the site because of its small size (0.01–0.03 mm). The residual films in the field could slowly break down into MPs and NPs with a combination of ultraviolet radiation and physio-chemical and biological possessions, resulting in MP and NP contamination in the soil [80][81][80,81].
Atmospheric deposition is another source of MPs and NPs entering surface soil. Atmospheric precipitation of plastic in the urban areas of Paris was around 2–35 particles m−2 days−1 [82]. Allen et al.’s [83] findings reported through air mass trajectory analysis that through atmospheric transportation, microplastic transport through the atmosphere at a distance of up to 95 km could reach and affect remote and sparsely populated areas. Microplastic detection in soil from remote/unsettled areas or restless high mountain areas has been reported [84].
Several studies documented that NPs are released from the degradation of plastic into the natural environment [85][86][87][85,86,87]. Studies by Ekvall et al. [88] revealed that MPs and NPs are produced during the mechanical degradation of daily use products. Lambert and Wagner [58] reported that MPs and NPs released in the degradation process of disposable cup lids constitute up to 1.26 × 108 particle mL−1 over 56 days. Mechanical and photo-oxidative degradation leads to the release of MPs and NPs into the natural environment. Literature reported that UV-light irradiation degraded PS components (PS foam, single-use plates, and coffee cup caps) into the soil environment [58] and is harmful to plants and soil organisms. Plastics appear in agricultural soil through different sources; however, the exact concentration of MPs and NPs from different sources are variable and as the study of the source is at an early stage, the exact source for the soil in various land uses is still unclear.
3. The Fate of Plastic in Agricultural Soil
Plastic has been reported both in surface and subsurface soil [84][89][90][91][92][84,89,90,91,92]. Different agricultural practices including tillage, irrigation, as well as soil organisms promote plastic transport in different soil layers [92][93][92,93]. Deep tillage, moldboard, and deep plowing methods disturb different layers of soil and promote the deep penetration of MP and NP in subsoils [92][94][95][92,94,95]. In addition, the pruning of rhizomes such as potatoes and carrots can also support MPs and NPs to migrate downward [96]. A current study found that wet-dry circles can promote MPs to move downward. Dry climate induces soil cracks, which could facilitate the plastic to move in deep soil [97]. Zubris and Richards [75] found evidence that fibers move downward but transport mechanisms are still unknown.
Cey et al. [98] reported that the average diameter of MPs that can leach down into deep soil of up to 70 cm is 3.7 mm. In addition, the penetration of water flow, such as rain or irrigation water from top to bottom in the ground, transmits MPs and NPs to the bottom with the soil vacuum and eventually leads to the groundwater [93][99][93,99].
Soil organisms are the most representative factor to transport MPs and NPs in deep soil [100]. There is growing evidence that soil organisms such as earthworms (Lumbricus terrestris), mites (Hypoaspis aculeifer, Damaeus exspinosus), and collembolans (Folsomia candida) can help migrate MPs and NPs from topsoil to deep soil [92][101][102][103][104][105][106][92,101,102,103,104,105,106]. Soil organisms can carry MPs and NPs through their casting, burrowing or ingestion, egestion and pushing, and also adhesion to their exterior [107]. Rilling et al. [108] reported that earthworms (Lambricus terrestris) added 35 to 73% of MPs (<50 μm and 63–150 μm in diameter) from surface debris to their burrows and also transported much smaller particles (<50 μm) into deep soil [92]. In topsoil polyethylene, MPs were penetrated up to 10 cm while NPs (710–850 μm) were mostly observed in the deepest layer. This result shows that earthworms transport plastic in terms of size and NPs penetrate deeper in the soil as compared to MPs. Earthworms can also pull large and microscopic plastic particles down into their burrows without eating the pieces of plastic [76]. The vertical movement of soil organisms creates micropores in the soil, which promotes the transportation of MPs and NPs by leaching. Yu et al. [103] reported that MPs are moved up to 50 cm below due to the bioturbation of earthworms in sandy soil and further leaching encouraged the MPs to reactivate even the largest part (250–1000 μm) which was found in a 60 centimeter-high leachate column.
3.1. Bioavailability
MPs and NPs present in soil in different forms and [109] their bioavailability depends on soil properties such as particle size, particle density, abundance/co-occurrence, chemical characteristics, and the specific characteristics of the receptor (plant or organism) [110][111][110,111]. The bioavailability of MPs increases with its size, and a wide range of organisms directly ingest it [112]. As their size fraction is similar to that of sediments and planktonic organisms, planktivores can directly eat MPs during normal food behavior as natural prey [111]. Soil chemical properties such as pH, sorption and adsorption of heavy metals, and their redox potential perform a vital role in the bioavailability of plastic in soil [113][114][115][113,114,115]. Furthermore, iron–manganese oxide-bound fractions of nickel (Ni), copper (Cu), chromium (Cr), and exchangeable carbonated-bound fractions in soil can be reduced by the presence of MPs [114]. The presence of high-density polyethylene (HDPE), polystyrene (PS), polyethylene (PE), and polylactic acid (PLA) increases the diethylenetriamine pentaacetate (DTPA) extractable Cd concentration of soil [116][117][118][116,117,118]. The presence of plastic in soil alters the bioavailability of other metals; arsenic bioavailability was limited in the presence of MPs [119]. MPs serving as the vector of heavy metals were reported in terrestrial systems. Adsorption behaviors and underlying mechanisms of HMs by MPs are critical to understand potential risks [120]. Earthworms increase the bioavailability of Cd in MP-enriched soils [96]. Wang et al. [117] reported the higher bioavailability of Cd in the presence of PLA in soil–plant systems as compared to PE.
MPs and NPs contain harmful substances including pesticides, polybrominated diphenyl ethers (PBDEs), endocrine-disrupting chemicals (EDCs), polycyclic aromatic hydrocarbons (PAHs), phthalates, and bisphenol-A that are transported to soil systems and leach down to subsoil based on temperature, ultraviolet radiation, soil pH, oxygen content, and dissolved organic matter content [55][107][121][122][55,107,121,122]. Sorption of EDCs and other substances on the surface of MPs and NPs can disrupt decomposition [123]. EDC threshold toxicity is difficult to measure because of non-monotonic dose effects and low dose effects [124]. Large amounts of toxic chemicals are associated with recycled plastics compared with virgin plastic [121]. PS MPs adsorb dibutyl phthalate, which has negative effects on green microalgae [125]. Wastewater and bio-solids have strong sorption of toxic substances from MP and NP particles [126][127][128][126,127,128]. A large amount of non-polar hydrophobic substances such as PCBs reportedly adsorb aged pellets of MPs compared to fresh pellets [129]. Heavy metals, dichlorodiphenyltrichloroethane (DDT), PAHs, and persistent organic pollutants (POPs) are adsorbed within MP and NP particles. Chemicals adsorbed on the surface of MPs and NPs are more toxic as compared to those directly coming from plastics. For example, it can be seen that the sorption of PS MP on metals is in the order of Pb2+ > Cu2+ > Cd2+ > Ni2+ [130]. Under the same conditions, the rate of Cr released from MPs was found to be faster than the rate of lead (Pb) release [131]. This may be due to different hydration ionic radii and differences in divalent cation complexing ability [132]. The composition of MPs influencing the adsorption, diffusion, and release of heavy metals depends on their morphology, specific surface area, surface charge, and porosity [133][134][135][133,134,135]. Wang et al. [116] reported that PE MPs adsorbed five pesticides (diflubenzuron, difenoconazole, carbendazim, malathion, and dipterex) in agricultural soil through exothermic and spontaneous processes; these results show that PE MPs can transfer different types of pesticides in agricultural soils.
The pH value can change the zeta potential of MPs or heavy metal precipitation, thereby increasing or decreasing the adsorption of certain metals. Generally, pH value increases with the decreasing zeta potential of MPs. However, if the MP’s zero-charge point is below the pH of the water, the MP charge will be negative. Thus, the electrostatic attraction between the metals and the polymer increases. In contrast, precipitation of some metals may occur in environments with a pH > 7. A recent study reported that pH increases with increasing the adsorption of Cu, Zn, Ni, Cd, Pb, and cobalt (Co) by MPs [132][136][137][138][139][140][141][142][132,136,137,138,139,140,141,142]. These increases may be due to an increased charge on MPs. In contrast, the adsorption of Cr+6 by MP is found to decrease with an increase in pH. This may be due to the relatively weak Coulombic interaction between the Cr+6 oxyanion form and the MP with reduced surface positive charge [143].
Plastic particles carry charge themselves which can enhance their adsorption in plant roots due to electrostatic attraction, affecting nutrient immobilization or photosynthesis processes [54]. The adsorption of Cd into PS MPs and PS NPs is associated with the reduction of the negative charge that they carry. Lian et al. [45] reported greater Cd bioaccumulation in wheat seedlings because of low Cd concentration in a PS NPs–Cd solution. PE MPs interact with heavy metals such as Cr (VI) in the existence of sodium dodecyl benzene sulfonate from Pentachlorophenol (PCPs) [144][145][146][147][144,145,146,147]. The adsorption of Cr (VI) were inhibited at a pH > 6, while it increases at a pH < 6 due to adsorption sites that available on PE MPs in increasing competition with sodium dodecyl benzene sulfonate. The four types of MPs [low-density polyethylene (LDPE), HDPE, polyvinyl chloride (PVC), and PE] were investigated using three types of heavy metals (Cd2+, Pb2+, and Cu2+). Plastic adsorbed on metal were found in the order of PE > PVC > HDPE > LDPE while metal adsorbed on MPs was Pb2+ > Cu2+ > Cd2+ [132]. The HDPE adsorption of Cd was increased with the increase of pH; in contrast, the efficiency reduced with the increase in salinity. Desorption showed a high tendency towards the adsorbed Cd [148] and posed a greater threat to the biotic environment [149].
3.2. Behaviour in Rhizospheric Soil
MPs and NPs adsorb contaminants in agricultural soil and may reach the rhizosphere zone. Different biochemical processes take place in rhizomes around the roots of plants [150]. Plants exude numerous substances such as exudates in the rhizosphere, which change the local environment of plants. Root exudates have been reported to contain phenolic mucilage, various amino acids, sugars, and ectoenzymes. Root exudates play a vital role in the plant rhizosphere to improve nutrient status as root exudates enhance soil structure and affect soil cation exchange capacity, pH, mineral degradation, microbial community, and sorption properties [151][152][151,152]. Abiotic stress caused by contaminants changes the root exudates. Vranova et al. [153] reported that under environmental stress, the quality of root exudates can increase 1000-fold from normal values. Abbasi et al. [151] reported that Pb, Cd, and Zn adsorbed on the surface of polyethylene terephthalate (PET) MPs in the wheat rhizosphere zone; the adsorption of Pb, Cd, and Zn in rhizosphere soil is associated with MPs and is available for a longer time to plants. Another study reported that in rice rhizosphere soil, PS MPs and polytetrafluorethylene (PTFE) particles combined with arsenic affect soil properties, available nutrients, soil enzymes, and microorganisms. PS MPs and PTFE combined with arsenic reduced the soil pH, bioavailable arsenic, available nitrogen, and phosphorus in the soil rhizosphere. Only arsenic increased soil organic matter while PS MPs and PTFE reduced soil organic contents. In addition, it also affected soil enzymes such as acid phosphatase, dehydrogenase, soil urease, protease, and peroxidase activity. PS MPs and PTFE and arsenic increased the abundance of Acidobacteria and Chloroflexi while reducing the abundance of Proteobacteria [119]. In the rhizosphere, the abundance of arbuscules, hyphae, and arbuscular mycorrhizal fungi was significantly increased with PS microfibers; this may depend on the alteration in soil structure and water dynamics by PS microfibers [154]. In contrast, PLA microplastic has significant adverse effects on arbuscular mycorrhizal fungi diversity and community structure, possibly due to toxicity associated with the biodegradation of PLA [155]. However, there is an urgent need to conduct research on the effect of MPs and NPs on soil properties, soil enzymes, soil microorganisms, and interaction with other contaminants in the plant rhizosphere.
3.3. Interaction with Soil Microbes
Soil microbes are key players in the biogeochemical cycling of elements and for the production of food. Better understanding the response of MPs to soil microorganisms will allow us to better predict the possible consequences as a result of MP pollution. MPs can act as a new host for microorganisms living in soil–plastic interfaces, which could lead to the formation of unique microbial communities [156].
Soil MPs and NPs change the diversity of bacterial and fungal communities. Some recent studies have reported that several types of MPs encourage and inhibit the bacterial community, and enzymatic activity such as Bacteroidetes and Actinobacteria increase their community on the surface of PE MPs [25][157][158][159][160][161][25,157,158,159,160,161]. Polyacrylic and polyester fibers decrease the metabolic activity of microbes [162]. In wheat soil systems, PVC and PE NPs shift microbial communities from Gram-positive to Gram-negative and also decrease xylosidase and β-glucosidase activity by 16–43% [163]. PVC increases Desulfobulbaceae and Desulfobacteraceae while decreasing Sedimenticolaceae and Chromatiaceae due to some antimicrobials which may be attributed to plastic additives [164]. In addition, PS microbeads inhibit Bacteroidetes, Proteobacteria, and Firmicutes due to the possible interaction with reduced soil nutrients as also shown in arsenic-polluted paddy soils [165]. MPs and NPs change soil properties and bacterial communities. Rhizobia can potentially change with changes in the soil matrix [166]. The ingestion of MPs by soil organisms could influence bacterial diversity; e.g., Zhu et al. reported that in the collembolan gut, MPs potentially enhanced bacterial diversity, possibly due to a move in feeding after MP exposure [102]. Some researchers have reported that some plastic microfilms such as PLA, PCL, PHA, PBAT, and starch-based biopolymers types have been approved as fixed C sources to enhance the concentration of fungal species, e.g., Fusarium, Aspergillus, and Penicillium [167][168][169][167,168,169]. Different sizes of plastic have different effects on microbes due to their change in surface area [78]. NPs (<0.1 μm) especially could enter the cell membrane and cause cytotoxic effects [170], due to the bioaccumulation in the cell of filamentous and yeast fungi [15][171][172][15,171,172]. The ability of NPs to enter and accumulate in soil organic debris causes biological effects on microbes, while NPs may be less important for altered soil properties. In addition, water-stable aggregates are important for microbial activity as PS fibers decrease water-stable aggregates, resulting in significant impacts on plant soil health. However, there is no data available on the effects of MPs and fungi on soil–plant systems [173]. Table 1 shows the impact of different types of plastics on soil microorganisms.
Table 1. The impact of plastics on soil microorganisms.
| Plastics | Level (%) | Effects on Microorganism | References | |
|---|---|---|---|---|
| PE | 1, 5, 10, 20 | Decreased xylosidase and β-glucosidase activity by 16–43% and MPs increased the soil microbial biomass (+43.6%). | [163] | |
| PVC | 1, 5 | Shown positive effects on acidobacteria, bacteriodietes, and hydrolase and urease enzymes while negative effects are shown on Sphingomonadaceae and the Fluorescein diacetate enzyme. |
[25][174] | [25,174] |
| PE | NA | MPs provided habitat to actinobacteria, bacteroidetes, Proteobacteria, gemmatimonadetes and Acidobacteria. Additionally, colonies of bacteria significantly varied in structure from those in the surrounding soil. |
[157] | |
| PE | 5 | In the fertilized soil, MPs significantly enhanced the bacterial and fungal community. MPs seem to indicate the selective impact on microbes and cause a serious hazard to biogeochemical cycles and microbes ecology. | [158] | |
| PE | 0.076 g kg | −1 | Increased Bacteriodietes, Acidobacteria, Nitrospirae, Gemmatimonadetes and diminished effect on nutrient cycling as well as positive effect on catalase urease enzymes. | [25] |
| PVC | 0.1 | Gut bacterial diversity increased and negative impact on soil macro- and micro-organisms. | [102] | |
| PS | 0.2, 0.4, 0.8 | Positive and negative effects of numerous Pro Firmicutes, teobacteria, and Bacteroidetes in various MP concentrations. | [165] | |
| PVC | NA | PVC increases Desulfobulbaceae, and Desulfobacteraceae and decreases Sedimenticolaceae and Chromatiaceae. | [164] | |
| PE, PLA | 0.1, 1 and 10 | MPs change the AMF diversity and structure that depend on their concentration level and type. Enriched with Ambispora (10% of PLA and PE), Archaeosporaceae (PLA 10%), and PLA have a negative impact on plant physiology i-e fresh/dry Biomass and Chlorophyll content. | [155] |
