Panax ginseng is a well-known medicinal plant that achieves strong resistance against plant pathogens while growing in the wild. Due to the high market demand for ginseng as a health food source, ginseng cultivation is prevalent in South Korea. However, continuous monocropping creates problems like irregular growth or vulnerability to crop diseases. Quorum sensing (QS) deals with the intracellular communication of bacteria and plays a role in dynamic changes in the soil microbiome. Here, we investigated how acyl-homoserine lactone (AHL) signaling molecules in QS (C8, C10, and C12) improve plant growth and induce shifts in the soil microbiome. To assess the effects, we recorded root and shoot growth of ginseng seedlings and checked the changes in the soil microbiome during different time points (0, 2, 4, and 8) after 8 weeks of growth. We observed that soils treated with N-decanoyl-L-homoserine lactone (C10) showed the most pronounced effects. Very striking was that C10 had the lowest alpha diversity. Using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2), we observed a high number of QS-related functional genes, with the highest count occurring in the untreated planted soil (W). Together with the known direct and beneficial effects of AHLs on plant development, AHLs treated mono-cropped soil showed trends in the microbiome community.
- ginseng
- soil microbiome
- quorum sensing
1. Introduction
2. Analysis on Results
2.1. Phenotypic Effect of the AHL Soil Treatments on Ginseng Seedlings

| Treatments | Shoot Length (cm) | Root Length (cm) | Fresh Shoot Biomass (g/plant) | Fresh Root Biomass (g/plant) | Dry Shoot Biomass (g/plant) | Dry Root Biomass (g/plant) |
|---|---|---|---|---|---|---|
| W | 4.87 ± 0.14 a | 14.67 ± 0.95 a | 0.45 ± 0.09 ab | 0.51 ± 0.03 a | 0.08 ± 0.01 a | 0.18 ± 0.01 a |
| C8 | 5.94 ± 0.83 a | 13.02 ± 1.04 a | 0.38 ± 0.06 a | 0.53 ± 0.06 a | 0.11 ± 0.02 ad | 0.19 ± 0.01 a |
| C10 | 6.05 ± 0.52 a | 13.54 ± 0.67 a | 0.61 ± 0.07 b | 0.83 ± 0.09 c | 0.15 ± 0.02 d | 0.32 ± 0.04 c |
| C12 | 4.62 ± 0.46 a | 13.27 ± 0.59 a | 0.39 ± 0.05 a | 0.53 ± 0.06 a | 0.09 ± 0.01 a | 0.20 ± 0.02 a |
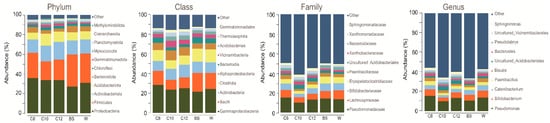
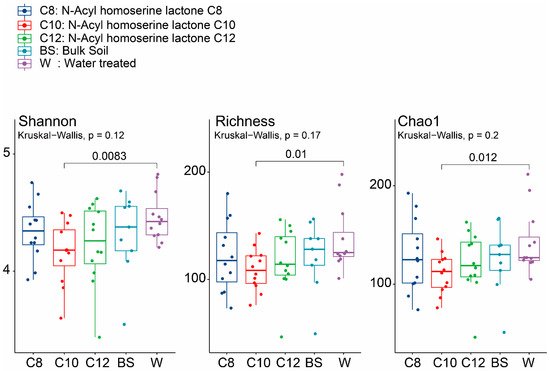
2.2. Microbial Community Shifts Involving AHL Treatments on Ginseng Soil
2.2. Microbial Community Shifts Involving AHL Treatments on Ginseng Soil
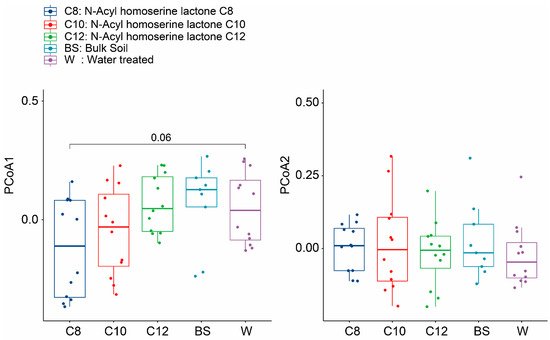
| DF | Sums of Sqs | Means Sqs | F. Model | R2 | Pr (>F) | ||
|---|---|---|---|---|---|---|---|
| Overall | Treatment | 4 | 1.8139 | 0.45346 | 1.1343 | 0.08025 | 0.021 * |
| Residuals | 52 | 20.7888 | 0.39978 | 0.91975 | |||
| Total | 56 | 22.6026 | 1.00000 | ||||
| 2 weeks | Treatment | 4 | 1.9865 | 0.49661 | 1.2304 | 0.32983 | 0.00 ** |
| Residuals | 10 | 4.0362 | 0.40362 | 0.67017 | |||
| Total | 14 | 6.0226 | 1.00000 | ||||
| 4 weeks | Treatment | 4 | 1.2441 | 0.31103 | 0.98797 | 0.30512 | 0.539 |
| Residuals | 9 | 2.8334 | 0.31482 | 0.69488 | |||
| Total | 13 | 4.0775 | 1.00000 | ||||
| 8 weeks | Treatment | 4 | 1.5079 | 0.37697 | 1.0388 | 0.34185 | 0.27 |
| Residuals | 8 | 2.9030 | 0.36288 | 0.65815 | |||
| Total | 12 | 4.4109 | 1.00000 |
3. Current Insights
References
- Wen, J.; Zimmer, E.A. Phylogeny and biogeography of Panax L. (the ginseng genus, Araliaceae): Inferences from ITS sequences of nuclear ribosomal DNA. Mol. Phylogenet. Evol. 1996, 6, 167–177.
- Yun, T.K. Brief introduction of Panax ginseng CA Meyer. J. Korean Med. Sci. 2001, 16, S3–S5.
- Kim, J.H. Cardiovascular diseases and Panax ginseng: A review on molecular mechanisms and medical applications. J. Ginseng Res. 2012, 36, 16.
- Ma, H.; Liu, D.; Sun, H. Deciphering microbiome related to rusty roots of Panax ginseng and evaluation of antagonists against pathogenic Ilyonectria. Front. Microbiol. 2019, 10, 1350.
- Wei, X.; Wang, X.; Cao, P.; Gao, Z.; Chen, A.J.; Han, J. Microbial community changes in the rhizosphere soil of healthy and rusty Panax ginseng and discovery of pivotal fungal genera associated with rusty roots. Biomed Res. Int. 2020.
- Atkinson, D.; Watson, C.A. The beneficial rhizosphere: A dynamic entity. Appl. Soil Ecol. 2000, 15, 99–104.
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001, 52 (Suppl. 1), 487–511.
- Song, C.; Zhu, F.; Carrión, V.J.; Cordovez, V. Beyond plant microbiome Composition: Exploiting microbial functions and plant traits via integrated approaches. Front. Bioeng. Biotechnol. 2020, 8.
- Dong, L.L.; Niu, W.H.; Wang, R.; Xu, J.; Zhang, L.J.; Zhang, J.; Chen, S.L. Changes of diversity and composition of fungal communities in rhizosphere of Panax ginseng. Zhongguo Zhong Yao Za Zhi 2017, 42, 443–449.
- Korenblum, E.; Dong, Y.; Szymanski, J.; Panda, S.; Jozwiak, A.; Massalha, H.; Meir, S.; Rogachev, I.; Aharoni, A. Rhizosphere microbiome mediates systemic root metabolite exudation by root-to-root signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 3874–3883.
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil. 2009, 321, 341–361.
- Lareen, A.; Burton, F.; Schäfer, P. Plant root-microbe communication in shaping root microbiomes. Plant Mol. Biol. 2016, 90, 575–587.
- Antoun, H.; Kloepper, J.W. Encyclopedia of Genetics; Academic: New York, NY, USA, 2001.
- Mohanram, S.; Kumar, P. Rhizosphere microbiome: Revisiting the synergy of plant-microbe interactions. Ann. Clin. Microbiol. 2019, 69, 307–320.
- Lei, H.; Liu, A.; Hou, Q.; Zhao, Q.; Guo, J.; Wang, Z. Diversity patterns of soil microbial communities in the Sophora flavescens rhizosphere in response to continuous monocropping. BMC Microbiol. 2020, 20, 272.
- Hartmann, A. Quorum sensing N-acyl-homoserine lactone signal molecules of plant beneficial Gram-negative rhizobacteria support plant growth and resistance to pathogens. Rhizosphere 2020, 16, 100258.
- Schenk, S.T.; Schikora, A. AHL-priming functions via oxylipin and salicylic acid. Front. Plant Sci. 2015, 5, 784.
- Hartmann, A.; Rothballer, M.; Hense, B.A.; Schröder, P. Bacterial quorum sensing compounds are important modulators of microbe-plant interactions. Front. Plant Sci. 2014, 5, 131.
- Liu, F.; Bian, Z.; Jia, Z.; Zhao, Q.; Song, S. The GCR1 and GPA1 participate in promotion of Arabidopsis primary root elongation induced by N-acyl-homoserine lactones, the bacterial quorum-sensing signals. Mol. Plant-Microbe Interact. 2012, 25, 677–683.
- Götz-Rösch, C.; Sieper, T.; Fekete, A.; Schmitt-Kopplin, P.; Hartmann, A.; Schröder, P. Influence of bacterial N-acyl-homoserine lactones on growth parameters, pigments, antioxidative capacities and the xenobiotic phase II detoxification enzymes in barley and yam bean. Front. Plant Sci. 2015, 6, 205.
- Shrestha, A.; Schikora, A. AHL-priming for enhanced resistance as a tool in sustainable agriculture. FEMS Microbiol. Ecol. 2020, 96, fiaa226.
- Yang, J.; Martínez, I.; Walter, J.; Keshavarzian, A.; Rose, D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 2013, 23, 74–81.
- Maquia, I.S.; Fareleira, P.; Videira E Castro, I.; Brito, D.; Soares, R.; Chaúque, A.; Ferreira-Pinto, M.M.; Lumini, E.; Berruti, A.; Ribeiro, N.S.; et al. Mining the Microbiome of Key Species from African Savanna Woodlands: Potential for Soil Health Improvement and Plant Growth Promotion. Microorganisms 2020, 8, 1291.
- Kämpfer, P.; Young, C.C.; Arun, A.B.; Shen, F.T.; Jäckel, U.; Rossello-Mora, R.; Rekha, P.D. Pseudolabrys taiwanensis gen. nov., sp. nov., an alphaproteobacterium isolated from soil. Int. J. Syst. Evol. Microbiol. 2006, 56, 2469–2472.
- Song, Q.; Song, X.; Deng, X.; Luo, J.; Wang, J.; Min, K.; Song, R. Effects of Plant Growth Promoting Rhizobacteria Microbial Inoculants on the Growth, Rhizosphere Soil Properties, and Bacterial Community of Pinus sylvestris var. mongolica Annual Seedlings. Res. Sq. 2020.
- Cipriano, M.A.; Lupatini, M.; Lopes-Santos, L.; da Silva, M.J.; Roesch, L.F.; Destéfano, S.A.; Freitas, S.S.; Kuramae, E.E. Lettuce and rhizosphere microbiome responses to growth promoting Pseudomonas species under field conditions. FEMS Microbiol. Ecol. 2016, 92, fiw197.
- Chen, L.; Hao, Z.; Li, K.; Sha, Y.; Wang, E.; Sui, X.; Mi, G.; Tian, C.; Chen, W. Effectsof growth-promoting rhizobacteria on maize growth and rhizosphere microbial community under conservation tillage in Northeast China. Microbial Biotechnol. 2021, 14, 535–550.
